Deepika Kumari1*, Ritu Kainth1, Sukhdeep Singh1, Parwinder Singh1, Ankush Kharyal2
Amar Shaheed Baba Ajit Singh Jujhar Singh Memorial College of Pharmacy,Ropar, Punjab, India
ISF College of Pharmacy Moga, Punjab, India
Received: 27 April, 2023; Processed: 22 May, 2023; Accepted: 29 May, 2023
Citation: Kumari, Deepika, RituKainth, Sukhdeep Singh, and Parwinder Singh, et al. “Exploring the Potential Benefits and Risks of 2,4-Thiazolidinediones (TZDs) in the Management of Diabetes and Related Conditions.” J Pharmacol Drug Deliv 1 (2023): 101. DOI: 10.59462/JPDD.1.1.101
Copyright: © 2023 Kumari D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
TZDs have been demonstrated to enhance insulin sensitivity and glucose absorption in muscle and adipose tissues, resulting in better glycemic control in type 2 diabetes patients. TZDs have also been demonstrated to have potential benefits in terms of lowering the risk of cardiovascular disease and improving lipid profiles. The potential benefits and hazards of a class of medications known as 2,4-thiazolidinediones (TZDs) in the treatment of diabetes and related disorders are discussed in this article. According to recent research, cardiovascular toxicity with rosiglitazone and a rise in bladder cancer with pioglitazone is no longer serious concerns. TZD use is also connected with major hazards, such as an increased risk of bone fractures, edema, and weight gain. Furthermore, TZDs have been linked to an increased risk of bladder cancer, and long-term use may result in liver damage. Overall, TZDs can be an effective tool in the treatment of diabetes and related disorders, but doctors must carefully balance the potential advantages with the hazards associated with their usage. Patients should be constantly monitored for side effects, and alternate treatment options should be sought when necessary.
Type 2 diabetes • Pharmacological effects • Clinical management • Risk factors • Pioglitazone • Rosiglitazone • Heart failure • Metabolic disorder • Bone fracture
Diabetes mellitus type 2 (T2DM) is a chronic metabolic disorder marked by insulin resistance and reduced insulin production. It is the most common kind of diabetes, accounting for around 90-95% of all cases. T2DM is a major global health issue, affecting an estimated 537 million people by 2021. T2DM prevalence is increasing, especially in low- and middle-income nations, as a result of ageing populations, urbanisation, and changing lifestyle factors such as poor diet and physical inactivity. T2DM is linked to several microvascular and macrovascular complications, including retinopathy, neuropathy, nephropathy, cardiovascular disease, and stroke, all of which can have a significant impact on quality of life and increase healthcare costs. T2DM must be diagnosed and handled as soon as possible to reduce the risk of complications [1-4]. 2,4-thiazolidinediones (TZDs) are a type of medicine that has been used to treat type 2 diabetes and other illnesses. They are well-known for their insulin-sensitizing properties, which increase glucose absorption and utilisation by body tissues. TZDs have also been demonstrated to alter adipose tissue metabolism, reduce inflammation, and enhance lipid profile, making them a potentially helpful therapy for metabolic diseases. Pioglitazone, rosiglitazone, and troglitazone are examples of medications in this class [5,6] . They function by raising the body’s sensitivity to insulin, resulting in increased glucose uptake and utilisation. Pioglitazone, rosiglitazone, and troglitazone are examples of medications in this class. TZDs have been studied for their potential benefits in treating illnesses other than diabetes, such as nonalcoholic fatty liver disease, cardiovascular disease, and polycystic ovarian syndrome. However, the use of TZDs has been linked to several hazards, including an increased risk of heart failure and bone fractures [7-9]. Fortunately, the Food and Drug Administration (FDA) has approved several new medications in recent years for the treatment of hyperglycemia in patients with type 2 diabetes mellitus. The most promising of these are the thiazolidinediones (TZDs) because data suggests that they may have a direct positive effect on some of these traditional and novel cardiovascular risk factors, independent of their hypoglycemic effects. Despite the potential benefits of TZDs, their usage has been limited due to the hazards associated with them. As a result, before using TZDs to treat diabetes or other illnesses, it is critical to carefully consider the potential advantages and hazards.
The purpose of this article is to investigate the potential benefits and hazards of TZDs in the treatment of diabetes and related disorders. We will conduct a literature evaluation on the efficacy of TZDs in diabetes management as well as their potential benefits in other conditions. In addition, we will cover the hazards connected with TZDs as well as the current guidelines for their use. Overall, the purpose of this review is to offer a thorough understanding of the possible benefits and hazards of TZDs in the treatment of diabetes and related disorders.
2,4-Thiazolidinediones (TZDs) are a type of oral diabetes medication used to treat type 2 diabetes. TZDs work by binding to the peroxisome proliferator-activated receptor gamma (PPAR-) in the nucleus of adipocytes and other cells. This increases insulin sensitivity and glucose uptake, which aids in the reduction of blood glucose levels [10]. PPAR- is a transcription factor that affects the expression of genes involved in glucose and lipid metabolism. TZDs activate PPAR- by attaching to a particular domain on the receptor, causing it to change conformation and interact with other proteins and DNA. This causes the transcription of genes involved in glucose uptake, such as Glut4, to increase, while inhibiting genes involved in gluconeogenesis, such as phosphoenolpyruvatecarboxykinase (PEPCK). TZDs have positive effects on lipid metabolism in addition to their effects on glucose metabolism. They boost the expression of genes involved in fatty acid uptake and storage, such as adipocyte fatty acid-binding protein (aP2) and fatty acid synthase (FAS), while suppressing the expression of genes involved in lipolysis, such as hormone-sensitive lipase (HSL). Overall, TZDs work by activating PPAR- , which improves insulin sensitivity, glucose absorption, and lipid metabolism. As a result, TZDs are an effective treatment for type 2 diabetes, particularly in patients with insulin resistance [11-14] as shown in Figure 1.
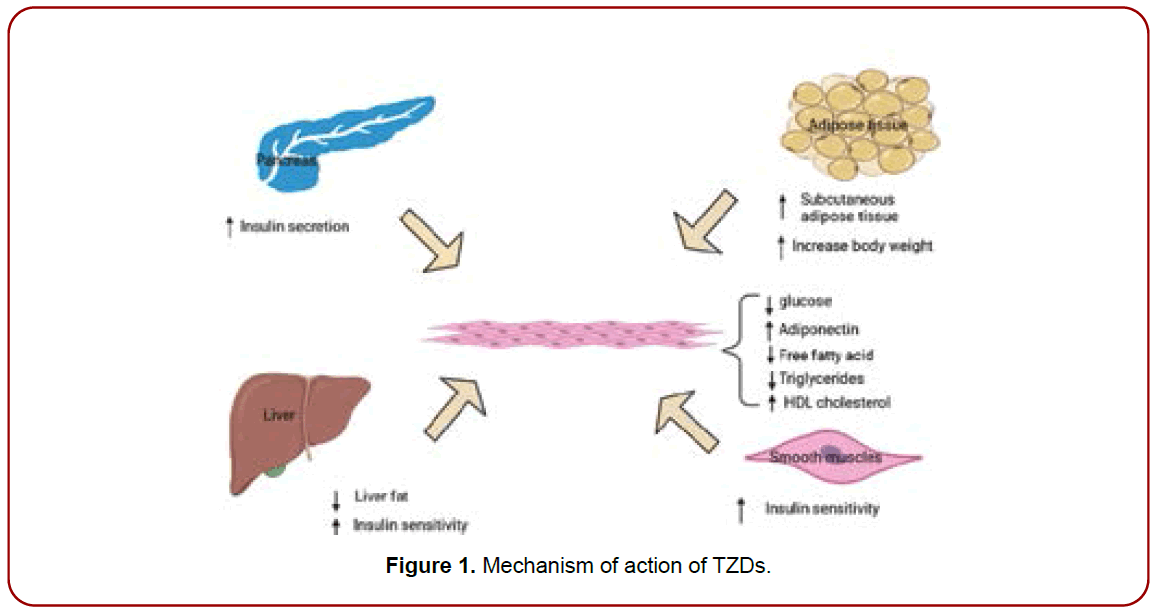
Figure 1. Mechanism of action of TZDs.
Several clinical trials have been conducted to assess the efficacy of TZDs in improving glycemic control in type 2 diabetic patients. Thiazolidinediones (TZDs) are a type of diabetic medicine that improves insulin sensitivity while decreasing insulin resistance. Pioglitazone and rosiglitazone are the two most often given TZDs. Clinical trials have been conducted to assess the efficacy of TZDs in improving glycemic control in type 2 diabetic patients. A 24-week randomised, double-blind, placebo-controlled trial with pioglitazone in 229 patients with type 2 diabetes, for example, found that pioglitazone lowered HbA1c levels by 1.5% when compared to placebo [15]. Rosiglitazone significantly lowered HbA1c levels by 1.3% compared to the placebo in a 26-week randomised, double-blind, placebo-controlled trial in 1,385 patients with type 2 diabetes [16].
A meta-analysis of 22 randomised controlled studies involving over 13,000 patients discovered that TZDs reduced HbA1c levels by 0.88% when compared to placebo or other diabetic treatments. This decrease was seen regardless of the patient’s age, gender, or baseline HbA1c level [17]. TZDs have been demonstrated to improve cardiovascular risk factors in addition to improving glycemic control. They can enhance lipid profiles, lower blood pressure, and have anti-inflammatory properties [18]. However, there are certain disadvantages to using TZDs. They have been linked to an increased risk of heart failure, especially in people who already have cardiovascular disease. They can also induce fluid retention and weight gain [19]. The effects of TZDs on lipid profiles and cardiovascular risk factors are summarised here. In a meta-analysis of 10 randomised controlled studies including over 4,000 patients, pioglitazone was demonstrated to significantly enhance HDL cholesterol levels by 4.4 mg/dL and decrease triglyceride levels by 31.5 mg/dL compared to placebo or other diabetic medicines. [20]. Although the effect on HDL cholesterol levels is not as constant as with pioglitazone, rosiglitazone has been demonstrated to enhance lipid profiles [21]. TZDs can improve endothelial function, lower blood pressure, and have anti-inflammatory properties [22]. The PROactive research, a large randomised controlled trial, found that pioglitazone reduced the incidence of nonfatal myocardial infarction, stroke, and mortality from cardiovascular causes in individuals with type 2 diabetes and a history of macrovascular disease [23]. TZDs, on the other hand, have been linked to an increased risk of heart failure, particularly in patients with previous cardiovascular disease [24].
There are several other classes of drugs used in the management of diabetes, here are some potential advantages of TZDs over all other drugs:
Improved insulin sensitivity
TZDs improve insulin sensitivity in adipose tissue, skeletal muscle, and liver, which leads to better glucose uptake and utilization by these tissues [19].
Preservation of beta-cell function
TZDs have been shown to preserve beta-cell function, which is important in maintaining normal glucose homeostasis in the long term [25].
Lipid-lowering effects
TZDs improve lipid profiles by decreasing triglycerides and increasing high-density lipoprotein (HDL) cholesterol levels [26].
Blood pressure control
TZDs have been shown to lower blood pressure in patients with hypertension, which is a common comorbidity in patients with diabetes [27].
Cardiovascular benefits
Some studies have suggested that TZDs may have cardiovascular benefits, such as reducing the risk of heart attack, stroke, and other cardiovascular events [28].
Cardiovascular risk
TZDs have been linked to an increased risk of cardiovascular events, especially in patients who already have cardiovascular disease. Several research has looked into the link between TZDs and cardiovascular risk. The PROactive trial, which assessed the cardiovascular safety of pioglitazone in individuals with type 2 diabetes and preexisting macrovascular disease, is one important study. The study discovered that pioglitazone lowered the risk of all-cause death, nonfatal myocardial infarction, and stroke. However, the pioglitazone group had an elevated risk of heart failure and edema [29]. The RECORD trial was another study that looked into the cardiovascular safety of rosiglitazone. There was no statistically significant difference in the risk of cardiovascular events between rosiglitazone and metformin or sulfonylureas in this study. However, the rosiglitazone group had an increased risk of heart failure and fractures [30]. Lincoff, et al. conducted a meta-analysis of randomised controlled trials to assess the cardiovascular safety of pioglitazone and rosiglitazone. The meta-analysis discovered that pioglitazone lowered the risk of all-cause mortality, nonfatal myocardial infarction, and stroke, whereas rosiglitazone did not affect this objective. Both pioglitazone and rosiglitazone, however, were linked to an increased risk of heart failure [31]. Singh, et al. discovered that TZDs were related to an increased risk of heart failure, myocardial infarction, and cardiovascular death when compared to other oral hypoglycemic medications in a meta-analysis of observational studies. The authors did warn, however, that the quality of data was limited due to the observational nature of the investigations [32].
Bone fracture
TZDs have been linked to an increased incidence of bone fracture, especially in women. Several research has looked into the link between TZDs and bone fracture risk. The ADOPT trial, which examined the long-term glycemic durability of rosiglitazone, metformin, and glyburide, is one significant study. The study discovered that women who took rosiglitazone had a greater rate of fractures than those who took metformin or glyburide [33]. Grey, et al. conducted a meta-analysis of randomised controlled trials to explore the relationship between TZDs and bone fracture risk. The meta-analysis discovered that TZDs were linked to an increased risk of fractures, especially in women. The risk of fractures increased with the use of TZDs for a longer period and at greater doses [34]. Schwartz, et al. discovered that TZDs were associated with an increased incidence of fractures, particularly in women, in a comprehensive review and meta-analysis of observational data. The authors discovered that the risk of fractures was highest during the first year of TZD usage and decreased as the length of use increased [35].
Weight gain
The use of TZDs has been linked to an increased risk of weight gain. Several research has looked into the link between TZDs and weight gain. The DREAM trial, which assessed the effect of rosiglitazone on the occurrence of diabetes in patients with impaired glucose tolerance, is one significant study. The study discovered that patients who took rosiglitazone gained more weight than those who received a placebo [36]. TZDs have been linked to an increased risk of weight gain. Several research has looked into the relationship between TZDs and weight gain. The DREAM trial, which examined the effect of rosiglitazone on the occurrence of diabetes in people with impaired glucose tolerance, is one significant study. The study discovered that patients who were given rosiglitazone gained more weight than those who were given a placebo [37]. Liu, et al. investigated the effect of adding pioglitazone to metformin therapy in type 2 diabetic patients. The study discovered that patients who took pioglitazone gained more weight than those who received a placebo, while the difference was minor [38].
Liver toxicity
Although they are generally regarded as safe and effective, there have been cases of liver toxicity as a result of their use. According to one study published in the journal Diabetes Care, pioglitazone, a commonly used TZD, was linked to an elevated risk of liver injury in type 2 diabetes patients. The researchers examined data from a major healthcare database and discovered that patients on pioglitazone had a greater risk of suffering liver injury than those who did not take the medicine. The researchers concluded that practitioners should be aware of this potential danger and actively follow patients for signs of liver damage [39]. Another study published in the journal Drug Safety looked at the occurrence of liver damage in patients using the TZD rosiglitazone. The study examined data from various clinical studies and discovered that rosiglitazone was associated with an increased risk of liver damage when compared to other diabetes medicines. The authors suggested that practitioners evaluate this potential risk when providing rosiglitazone to individuals with type 2 diabetes [8]. Furthermore, the US Food and Drug Administration (FDA) has issued cautions about the potential risk of liver toxicity associated with TZDs. The FDA suggests that clinicians monitor liver function in TZD patients and consider discontinuing the medication if signs of liver impairment emerge [14] While TZDs are generally thought to be safe and effective in the treatment of type 2 diabetes, there is a risk of liver damage with their use. Clinicians should be aware of this risk and thoroughly monitor patients for symptoms of liver damage.
Bladder cancer
Concerns have been raised about the link between thiazolidinediones (TZDs) and bladder cancer, notably pioglitazone. Long-term pioglitazone use has been linked to an increased risk of bladder cancer in several studies. A 2016 study published in the British Medical Journal examined data from over 145,000 types 2 diabetes patients and discovered that long-term usage of pioglitazone was connected with an elevated risk of bladder cancer. The study discovered that people who had taken pioglitazone for more than two years had a 63% higher risk of developing bladder cancer than those who had never taken the medicine. Patients who had taken the highest cumulative dose of the medicine were in the greatest danger [40]. Another study published in the journal Diabetes Care in 2017 indicated that using pioglitazone increased the risk of bladder cancer. The researchers examined data from a major healthcare database and discovered that people on pioglitazone had a greater risk of developing bladder cancer than those who did not take the medicine. According to the authors, doctors should be aware of this potential danger and carefully weigh the benefits and hazards of pioglitazone therapy in individual patients [41]. In response to these concerns, the United States Food and Drug Administration (FDA) has issued warnings about the risk of bladder cancer associated with pioglitazone use. The FDA suggests that healthcare practitioners carefully examine the benefits and hazards of pioglitazone treatment in individual patients and that patients be informed of this potential risk [42].
Macular edema
Thiazolidinediones (TZDs), which include pioglitazone and rosiglitazone, have been linked to an increased risk of macular edoema, a disorder characterised by swelling in the central region of the retina that can result in visual loss. A 2013 study published in the journal Ophthalmology discovered that patients on pioglitazone had a much higher chance of developing macular edoema than those who did not take the medicine. The study examined data from over 70,000 diabetic patients and discovered that the risk of macular edoema was more than twice as high in people using pioglitazone as in those who did not [43]. Similarly, a 2014 study published in the journal JAMA Ophthalmology discovered that patients on rosiglitazone had a much higher incidence of macular edoema than those who did not take the medicine. The study analysed data from over 100,000 diabetic patients and discovered that the incidence of macular edoema was around three times higher in people taking rosiglitazone compared to those who did not take the treatment [44]. The exact mechanism through which TZDs may raise the risk of macular edoema is unknown, however, it could be connected to the medications’ effects on fluid balance and inflammation in the retina [45]. Clinicians should be aware of this potential danger and closely monitor TZD patients for indications of macular edoema, such as hazy vision or visual distortions. Patients should also be aware of this potential danger and advised to notify their healthcare practitioner if they experience any visual complaints.
The current guidelines for the use of TZDs in diabetes mellitus include the following:
American Diabetes Association (ADA) Standards of Medical Care in Diabetes – 2022
When other oral diabetes drugs have failed to reach glycemic objectives, the ADA recommends TZDs as second or third-line therapy in type 2 diabetes. Because of concerns about cardiovascular risk with rosiglitazone, pioglitazone is preferred. The ADA also advises monitoring for TZD side effects such as fluid retention and heart failure [46].
European Association for the Study of Diabetes (EASD) Clinical Practice Guidelines for Diabetes – 2020
When metformin alone fails to achieve glycemic objectives in type 2 diabetes, the EASD recommends TZDs as a second-line therapy. Pioglitazone is favoured over rosiglitazone due to concerns about cardiovascular safety with rosiglitazone. The EASD also advises monitoring for TZD side effects such as heart failure and bone fractures [47].
International Diabetes Federation (IDF) Clinical Practice Recommendations for Managing Type 2 Diabetes – 2021
When other oral diabetes drugs have failed to reach glycemic objectives, the IDF recommends TZDs as a second or third-line therapy in type 2 diabetes. Because of concerns about cardiovascular risk with rosiglitazone, pioglitazone is preferred. The IDF also advises keeping an eye out for TZD side effects such as weight gain and oedema [48].
Here are some future research directions to improve the efficacy and safety of TZDs in the management of diabetes mellitus and related conditions:
Development of Selective PPARγ Modulators (SPPARγMs)
The peroxisome proliferator-activated receptor (PPAR) is activated by TZDs and controls glucose and lipid metabolism. However, PPAR activation might have negative consequences. SPPARMs are a novel class of drugs that selectively activate specific PPAR target genes, lowering the likelihood of side effects. Several SPPARMs are now in development and may outperform TZDs in terms of efficacy and safety [49].
Combination therapy with other antidiabetic drugs
TZDs can be used in conjunction with other diabetes medications to improve glucose control while lowering the dose and risk of side effects. Combination therapy with a sodium-glucose cotransporter2 (SGLT2) inhibitor, for example, has been demonstrated to enhance glycemic control, reduce body weight, and lower blood pressure in T2DM patients [50].
Personalized medicine
Individual responses to TZDs differ, and genetic factors may play a role in this diversity. Personalised medicine techniques that use genetic testing to identify patients who are likely to react to TZDs have the potential to improve efficacy while lowering the risk of unwanted effects [51].
Development of new TZD analogs
New TZD analogues are being developed to retain the therapeutic effects of TZDs while lowering the likelihood of unwanted effects. Some analogues, for example, have been engineered to be more selective for PPAR and have a shorter half-life, potentially lowering the likelihood of unwanted effects [52]. Future research on TZDs and related chemicals should aim on enhancing efficacy and safety through the development of SPPARMs, combination therapy, personalised medicine methods, and novel TZD analogues. These techniques have the potential to improve T2DM control while lowering the risk of unwanted consequences [52].
2,4-Thiazolidinediones (TZDs) have been demonstrated to effectively control blood glucose levels in type 2 diabetic patients. They are, however, linked to potential adverse effects such as weight gain, fluid retention, bone fractures, heart failure, and liver issues. Another factor to consider when using TZDs is the possibility of drug interactions. When used with insulin or sulfonylureas, TZDs can influence the metabolism of other medicines, including oral contraceptives, and may raise the risk of hypoglycemia. While TZDs can be an effective therapeutic choice for some individuals, their usage should be carefully reviewed and managed, taking into account each patient’s risk factors, medical history, and preferences. As a result, in the management of diabetes and related disorders, a personalised therapy approach is critical. Such an approach should entail patients and healthcare providers working together to identify the best treatment plan that balances the possible advantages and dangers of TZDs, as well as other treatment options. TZDs have been explored for their potential benefits in illnesses other than diabetes management, such as non-alcoholic fatty liver disease, polycystic ovarian syndrome, and Alzheimer’s disease. More research, however, is required to assess the efficacy and safety of TZDs in these circumstances. TZDs are not suitable for all people with type 2 diabetes. TZDs should be avoided by people who have a history of heart failure, liver illness, or bladder cancer. Furthermore, TZDs should not be used during pregnancy or in people who have a history of bone fractures or osteoporosis. Overall, using TZDs to address diabetes and related disorders necessitates careful assessment of potential benefits and hazards, as well as unique patient variables and preferences. Close monitoring of TZD patients is required to reduce the risk of side effects and ensure optimal treatment outcomes.