Francesco Cavalli1*, Josuel Ora2 and Paola Rogliani3
Division of Respiratory Medicine, University Hospital “Tor Vergata”, Via Montpellier, 1 – 00133 Rome, Italy
Division of Respiratory Medicine, University Hospital Tor Vergata, Rome, Italy
Department of Experimental Medicine, University of Rome “Tor Vergata”, Rome, Italy
Received: 09 December 2023; Processed: 23 December 2023; Accepted: 31 December 2023
Citation: Cavalli F, Ora Josuel and Rogliani Paola. “Idiopathic Pulmonary Fibrosis and Alpha 1 Antitrypsin Deficiency: A Case Report of a Patient Undergoing Lung Transplantation and Intravenous Augmentation Therapy.” J Pulm Respir Dis (2023): 101. DOI: 10.59462/JPRD.1.1.101
Copyright: © 2023 Cavalli F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Idiopathic Pulmonary Fibrosis (IPF) is a chronic interstitial lung disease characterized by progressive lung scarring and the pathological hallmark of usual interstitial pneumonia (UIP). On the other hand, Alpha-1 antitrypsin deficiency (AATD) is a genetic disorder predisposing individuals to lung and liver diseases. Despite its rarity in literature, the relationship between IPF and AATD remains unclear. While IPF often necessitates lung transplantation in advanced stages, traditional AATD management involves supportive therapy. However, the introduction of supplemental alpha- 1-antitrypsin (AAT) derived from pooled human plasma has shown promise in retarding lung function decline. Notably, post lung transplantation, the genetic basis of AATD-related lung disease persists, potentially exposing patients to disease recurrence.
Here, we present a clinical case of a patient initially diagnosed with IPF who subsequently underwent double lung transplantation and was later diagnosed with AATD. This case study prompts inquiry into critical unresolved questions: the potential benefits of post-transplant augmentation therapy for AATD patients and the potential association between AATD and pulmonary fibrosis. The intricate interplay between these two conditions underscores the need for further investigation to enhance our understanding and improve patient outcomes.
Alpha1-Antitrypsin Deficiency (AATD) • Idiopathic Pulmonary Fibrosis (IPF) • Forced Vital Capacity (FVC) • Carbon Monoxide Diffusion Capacity (DLCO) • Six-Minute Walking Distance (6MWD)
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease characterized by intense alveolar inflammation, destruction of the pulmonary vascular bed and subsequent fibrosis of the pulmonary interstitium. It is characterized by progressive worsening of dyspnoea and lung function and is associated with a poor prognosis. Prevalence estimates for IPF have varied from 2 to 29 cases per 100,000 in the general population [1].
Alpha-1 antitrypsin deficiency (AATD) is one of the most common metabolic disorders in persons of northern European heritage, occurring in approximately one in 5,000-7,000 individuals in North America and one in 1,500-3,000 in Scandinavia. AATD also occurs (in lower frequencies) in all other racial subgroups worldwide [2]. It is a co-dominant inherited disorder characterized by the reduction of AAT, a strong plasma inhibitor of various proteases. Several variants are known. Normal individuals are described as MM, and disease is most frequently seen when S and/or Z alleles are present. AAT is synthesized by mononuclear hepatocytes and phagocytes, is secreted by the liver, and circulates in the plasma with a half-life of 5 days [3]. In AATD, proteases without proteolytic activity, predominate in the lower respiratory tract, leaving the lung vulnerable to parenchymal destruction and typically favoring the development of panacinar emphysema [4].
When IPF and AATD patients develop end-stage lung disease, lung transplantation is the only treatment option available, and this can improve lung physiology and patient health status. AAT augmentation therapy plays an important role in postponing lung transplantation, but its role following lung transplantation is unclear and needs to be explored [5].
Below is a description of a clinical case of a patient suffering from IPF, undergone to double lung transplant, who was later diagnosed an AATD and then was treated with AAT augmentation therapy.
A bus driver of 54-year-old, non-smoker man, with worsening dyspnea, was reported to our pulmonary fibrosis center for clinical evaluation of interstitial lung disease, in January 2017. In his history, no relevant exposures to either organic or inorganic dusts have been reported, nor have significant information been reported in family history or allergies. As well as in the clinical history beyond arterial hypertension, dysthyroidism and type 2 diabetes, no clinical symptoms, or any signs of disease suggestive of connective tissue disease were reported. About six months earlier, following a flu-like episode, the patient underwent to a full radiological evaluation that include initially chest X-ray showing interstitial signs associated to bilateral bronchiectasis., and then a high-resolution computed tomography (CT).
At clinical evaluation the patient was in fair condition, with normal blood pressure, heart, and respiratory rate. At physical examination he presented bibasal teleinspiratory velcro-like crackles, no clubbing, nor cyanosis. Mild normocapnic hypoxemia was detected at the arterial blood gas analysis. Pulmonary function test (PFT) and diffusing lung capacity for carbon monoxide (Dlco) showed an initial restrictive pattern to the lung and a moderate alteration in the diffusion capacity (Table 1). Serological features of collagen vascular diseases were negative. A prevalence of alveolar macrophages was detected at the cytopathological examination of bronchoalveolar lavage fluid (BALF). BALF microbiology was negative, including opportunistic infections such as Pneumocystis.
| Pre-lung transplantation | ||||||||||||||
| DATE | FVCL | FVC% | FEV1L | FEV1% | FEV1/ FVC | RVL | RV% | TLCL | TLC% | Dlco (mmol/ min*Kpa) | Dlco% | 6MWD | 6MWD (NADIR%) | Oxygen (l/min) |
| 20-02-2017 | 1,69 | 45 | 1,30 | 42 | 77,35 | 0,87 | 41 | 2,56 | 42 | N.A. | N.A. | N.A. | N.A. | N.A. |
| 02-08-2017 | 1,85 | 49 | 1,58 | 52 | 85,67 | 1,18 | 56 | 3,05 | 50 | 2,75 | 31 | 525 | 90 | 0 |
| 20-02-2018 | 1,70 | 45 | 1,45 | 48 | 85,20 | 1,20 | 57 | 2,92 | 48 | 2,63 | 30 | 450 | 85 | 0 |
| 06-12-2018 | 1,88 | 55 | 1,49 | 53 | 79,16 | 1,42 | 68 | 3,29 | 58 | N.A. | N.A. | N.A. | N.A. | N.A. |
| 08-07-2019 | 1,06 | 31 | 0,76 | 27 | 71,51 | 1,07 | 51 | 2,44 | 43 | N.V. | N.V. | 405 | 83 | 0 |
| 07-10-2019 | 1,33 | 36 | 0,86 | 29 | 64,78 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | 420 | 84 | 3 |
| Post-lung transplantation | ||||||||||||||
| DATE | FVCL | FVC% | FEV1L | FEV1% | FEV1/ FVC | RVL | RV% | TLCL | TLC% | Dlco (mmol/ min*Kpa) | Dlco% | 6MWD | 6MWD (NADIR%) | Oxygen (l/min) |
| 06-11-2020 | 2,13 | 63 | 1,93 | 71 | 90,61 | 1,71 | 81 | 3,86 | 68 | N.A. | 71 | 420 | N.A. | 0 |
| 16-04-2021 | 1,79 | 53 | 1,55 | 57 | 86,59 | 0,67 | 31 | 2,36 | 41 | N.A. | 39 | 420 | N.A. | 0 |
| 11-04-2022 | 2,02 | 52 | 1,52 | 50 | 75,20 | 0,85 | 38 | 2,87 | 47 | 4,92 | 62 | 375 | 93 | 0 |
| 17-10-2022 | 2,01 | 55 | 1,54 | 53 | 76,55 | 1,44 | 64 | 3,44 | 56 | 5,45 | 65 | 450 | 94 | 0 |
| 27-03-2023 | 1,83 | 51 | 1,29 | 45 | 70,25 | 1,71 | 76 | 3,65 | 60 | 5,35 | 64 | N.A. | N.A. | N.A. |
| 29-12-2023 | 2,09 | 54 | 1,59 | 53 | 76,10 | 0,95 | 42 | 3,04 | 50 | 5,87 | 74 | 360 | 97 | 0 |
Table 1. General respiratory function data and six-minute walking test (6MWTD: Six-minute walk test distance; Dl’co: Diffusing capacity for carbon monoxide; FEV1: Forced expiratory volume in 1 second; FVC Forced vital capacity; N.A.: Not available; N.V.: Not valuable; RV: Residual volume; TLC: Total lung capacity; VC: Vital Capacity)
The case was discussed at the multidisciplinary discussion (MDD) and according to the clinical presentation, the lab tests and the CT pattern classified as radiologically indeterminate for IPF, it was decided to treat with prednisone, 25 mg daily for 4 weeks, tapered to a maintenance dose of 10 mg daily and torevaluate. After two months, clinical conditions and chest CT scan did not show any improvement, so an awake VATS lung biopsy was performed. A histopathological pattern of UIP was described [1]. A final diagnosis of IPF was achieved by a subsequent MDD and a treatment with nintedanib 150 mg bid was started. After three and then six months the patient was re-evaluated; he presented a progressive decline of clinical condition, along with PFT decline and progressive respiratory failure, hence he was screened for inclusion in transplant list and after three years, in June 2020 the patient received a double lung transplant.
During routine follow up the patient was re-evaluated, according to an internal protocol which also included the dosage AAT highlighting a low cut-off of 83.40 mg / dl. Then, a genetic evaluation was conducted with a detection of heterozygous PiMS phenotype: the deficient allele S (p.E288V - E264V - c.863A> T rs17580). The data was consistent with the phenotype obtained by isoelectric focusing (IEF) and with the plasma levels of AAT measured on the sample, in agreement with an intermediate deficit of AAT. Based on the anamnestic, clinical and laboratories characteristics a specific treatment with human alpha1- proteinase inhibitor was started in October 2020. The treatment was initiated with the approved dose of 60 mg/ kg weekly intravenous (IV).
About a year after the transplant, the patient’s general conditions worsened. PFT showed a progressive decline in respiratory function (Table 1) associated with the appearance of nodular shaped ground glass opacities and consolidations at CT chest. The BAL exam did not reveal acute infections for common and opportunistic germs; transbronchial biopsies ruled out rejection disease. Following the appearance of viny red, painful nodular lesions (April 2021) in the lower limbs and feet, in the suspicion of Kaposi’s sarcoma, the patient did a dermatological examination and then a skin biopsy which confirmed the clinical suspicion of Kaposi’s sarcoma (June 2021). The immunosuppressive therapy was partially modified, and the patient was referred to the oncologist to start specific treatment. After targeted therapy with chemotherapeutic and immune modulatory agents, Kaposi’s sarcoma resolved.
The patient is currently being followed in our department. Almost three years after the transplant, he is in fair condition, the trend of pulmonary function tests, after an initial decline correlated to Kaposi’s sarcoma lung involvement, has essentially stabilized (Table 1). The radiological features are improved. He is continuing augmentation therapy with alpha-1 proteinase inhibitor at the initially established dosage without complications. He is maintaining plasma concentration of AAT higher than 110 mg/dl.
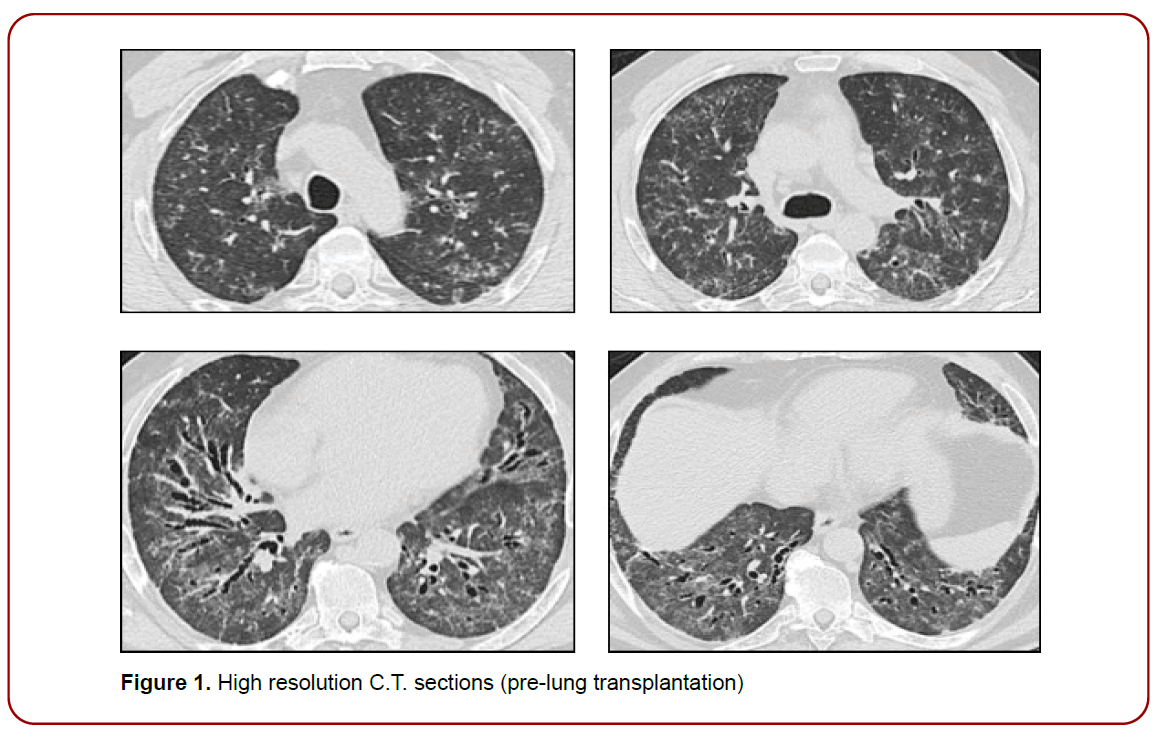
Figure 1. High resolution C.T. sections (pre-lung transplantation)
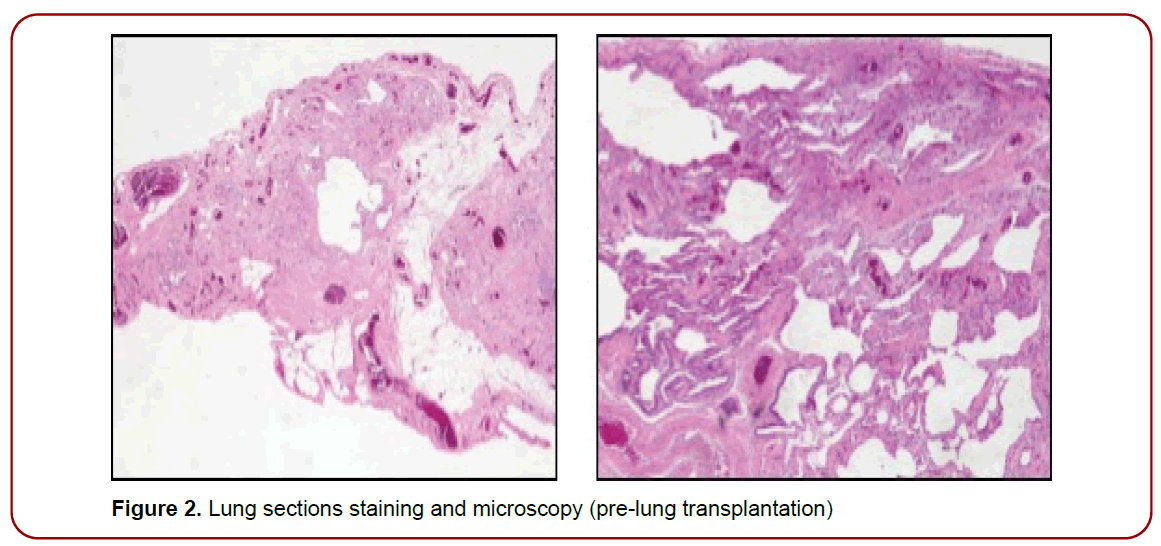
Figure 2. Lung sections staining and microscopy (pre-lung transplantation)
This case reveals an unusual manifestation of IPF in a young man, necessitating a lung biopsy due to the rapid progression of symptoms. The complexity escalated with the post-transplant discovery of AATD and the subsequent development of Kaposi sarcomas.
Atypical HRCT features frequently accompany a histopathological pattern of UIP/IPF (approximately 30%) [6] though the probability of IPF is notably higher in individuals aged 55 and older [7].
The biopsy provided a conclusive diagnosis and enabled the initiation of a therapeutic regimen aimed at slowing the disease progression. However, due to the patient’s age, lung transplantation became an inevitable course of action.
The discovery of a heterozygous PiMS phenotype, linked to decreased AAT dosage, gives rise to two distinct inquiries: first, is there a pathogenic connection between IPF and AATD? Second, how should we strategize the management of this case given the available evidence?
AAT serves as a crucial anti-protease within the lung, while also exerting noteworthy anti-inflammatory impacts on diverse cell types and regulating inflammation prompted by both host and microbial factors. Consequently, it assumes a pivotal role in modulating essential immune cell functions, safeguarding the lungs against harm from proteases and inflammation [8]. The PiS allele codes for approximately 60% of the normal serum levels of the protein. It’s important to note that serum levels of AAT in individuals with PiM and PiS alleles can exhibit overlap, and the PiMS phenotype has yet to be fully characterized [9]. On the other hand, although we found a lower level of AAT, it is possible to assume that our patient with the PiMS phenotype would normally have a lower level of AAT than that measured during hospitalization due to his chronic inflammatory condition. In addition, the physiological activity of these aberrant protease inhibitors could differ from the normal protein, in a way that contributes to the pathology beyond the pure quantitative deficit.
The prevalence of AATD among IPF subjects remains uncertain; while some authors have reported a higher occurrence of the MZ phenotype in IPF subjects when compared to healthy individuals, this result has not been consistently confirmed by other studies [10,11].
AATD disrupts the balance between pro-inflammatory and anti-inflammatory factors, and it can be hypothesized that in certain patients, it might contribute to the predisposition for inflammatory lung diseases that subsequently manifest as pulmonary fibrosis. Current theories regarding the pathogenesis of pulmonary fibrosis often revolve around an aberrant inflammatory response to unidentified triggers. In this context, inflammation and immunoregulatory cells infiltrate the interstitium and alveolar space, resulting in damage to epithelial and endothelial cells and instigating collagen production by fibroblasts [12]. While the potential influence of AATD and its phenotypes on the pathogenesis of IPF remains speculative, demanding further investigation, in our patient’s case, it prompted a practical decision on whether to initiate augmentation therapy or not.
Only a limited number of studies have delved into the post-transplant outcomes among individuals with AATD, where the hypothesis of a heightened risk of proteolytic damage has been postulated [13]. In relation to the influence of augmentation therapy on post-lung transplant survival, there is a lack of evidence, thereby contributing to the absence of a conclusive consensus regarding its applicability. Some smaller-scale studies have suggested that AATD subjects, who persist with replacement therapy after transplantation, manifest survival rates that are akin to those seen in the absence of AATD-associated COPD [14]. Further complicating the interpretation of this limited data, Conrad et al. conducted a retrospective analysis employing an extensive repository of transplant patient data [15]. Their findings, somewhat unexpectedly, showed that subjects who underwent augmentation therapy prior to lung transplantation exhibited a noteworthy decrement in survival rates after a decade, a trend contrasting against both the non-augmentation recipients and those with COPD.
About Kaposi’s sarcoma, this is a pathological entity closely associated with solid organ transplantation but remains extremely rare after lung transplantation. The course of the malignancy in the setting of lung transplant is limited to the experience of few existing case reports. Thus, the long-term prognosis of Kaposi’s sarcoma is poorly defined in these patients [16]. In this case a combination of chemotherapy and immunotherapy, along with a reduction of immunosuppressive therapy, resulted effective.
Lung disease associated with AATD can evolve in different ways, (not exclusively into severe forms of chronic obstructive pulmonary disease), and predisposing factors are not fully understood, making its early diagnosis relevant [17]. After lung transplantation, the genetic cause of AATD related lung disease remains and patients may risk disease recurrence [18]. Augmentation therapy for AATD should therefore be indicated in all these cases. However, it is fair to point out that to date, there is still no evidence on the matter and there is no univocal consensus for its use or not.
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
None declare.
This clinical case highlights two issues: the possible benefit of augmentation therapy, in patients with AATD, after lung transplantation and the possible association between AATD and pulmonary fibrosis. Furthermore, appear evident how delicate is the management of patients undergoing to lung transplant and the different possible complications related to it, especially between period immediately after surgery and one year.