Sandra Maeso Méndez1,2, Eider Jauregui Benito1, Laura Costa Serra1, Andrea Gainzarain Serna1, Annabel Prigent Diaz1, Carlos Sola Sarabia4, Mikel Ogueta Lana5, Rut Gago Martín3 and Ignacio Díez López2,6,7*
Department of Pediatrics, Áraba University Hospital, OSI Araba Vitoria-Gasteiz, Álava, Spain
Department of Childhood Endocrinology, Pediatrics Service, Áraba University Hospital, OSI Araba Vitoria-Gasteiz, Álava, Spain.
CS Zabalgana OSI Araba Vitoria-Gasteiz, Álava, Spain.
Department of Health, Basque Government. Spain.
Department of Health and Quality Information, Basque Government of Spain
Department of Pediatrics. UPV-EHU. Vitoria-Gasteiz, Álava, Spain.
BIOARABA. Investigation Institute, Child and youth growth and metabolism group, Vitoria-Gasteiz, Álava, Spain.
Received: 13 May 2024; Accepted: 22 May 2024; Published: 31 May 2024
Citation: López, Ignacio Díez, et al. “Influence Use of the Bacillus Calmette- Guerin Vaccine on the Incidence Type 1 Diabetes Mellitus Onset in a Child and Adolescent Population.” J Diabet Clin Endocrinol (2024): 105. DOI: 10.59462/JDCE.2.1.105.
Copyright: © 2024 Lopez ID. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction: Type 1 diabetes mellitus is a chronic disease of autoimmune etiology that is highly prevalent in childhood. In the last decade, the possibility of using the Bacillus Calmette-Guérin vaccine for the treatment of this disease has been studied thanks to its immunomodulatory action.
Objectives: The objective of this work is to carry out a systematic review of the available scientific evidence on the relationship between the tuberculosis vaccine and the treatment of diabetes in childhood. Secondarily, the aim is to study the incidence of Type 1 Diabetes Mellitus in a cohort of children from the Basque Country according to whether or not they have received the vaccine.
Methods: An exhaustive bibliographic search has been carried out on the evidence available to date. For the epidemiological study in Euskadi, a comparison of accumulated incidences between vaccinated and unvaccinated population groups was made.
Results: According to the literature analyzed, there is more and more evidence about the possible use of the tuberculosis vaccine in Type 1 Diabetes Mellitus. The results of our study were not conclusive, but we believe that it is necessary to continue with long-term studies to eliminate biases.
Conclusion: The Bacillus Calmette-Guérin vaccine could be used for the treatment of autoimmune, allergic, infectious or oncological processes, although more studies are still necessary in this regard.
BCG vaccine • Diabetes Mellitus type 1 • Mycobacterium tuberculosis
Diabetes Mellitus type 1 (DM-1) is a very common chronic disease in childhood. It is an autoimmune disease in which T lymphocytes destroy the beta cells of the pancreas, generating a decrease in insulin levels. Its incidence in Spain is around 10-12 cases per 100,000 children under 14 years of age, with two characteristic age peaks: between 4 and 6 years and between 10 and 14 years [1]. In the specific case of the Basque Country, its incidence in children under 14 years of age ranges between 9.5 and 16 cases per 100,000 children, being minimum in the age group of 0-5 years and maximum between 13-14. years. Furthermore, in the pediatric population, no differences in incidence are found in terms of sex [2].
In the last decade, the possibility that the Bacillus Calmette- Guérin (BCG) vaccine could be an effective alternative for the treatment of DM-1 through its immunomodulatory action has been studied. On the one hand, it stimulates the production of tumor necrosis factor (TNF), which “destroys” autoimmune T lymphocytes. And on the other hand, it increases the production of regulatory T lymphocytes through epigenetic modifications. In addition, it is capable of generating changes in glucose metabolism to ultimately reduce blood glucose levels. BCG was eliminated from the vaccination schedule of the Basque Country on January 1, 2013, being the only Spanish Autonomous Community that used it for all newborns with a single dose at one month of age.
We believe, therefore, that we have a good scenario to assess whether or not its use influences the incidence of DM-1. Therefore, the objective of the present study is to analyze the possible immunomodulatory effect of BCG on DM-1 in the pediatric population of the Basque Country after its withdrawal from the childhood vaccination schedule, leaving the entire cohort of minors born after 2013. not exposed to BCG. These authors have published previous comparison data between fully exposed cohorts and a partially unexposed one in Gago et al in Bol S Vasco- Nav Pediatrician 2022 [3] comparing 6 years before BCG withdrawal vs. 6 years after. Part of the data used in this original comes from this work.
First, a bibliographic review was carried out to compile the most relevant information on the relationship between the immunomodulatory effect of the BCG vaccine and DM-1. To search for bibliographic documents, mainly scientific articles, national and international databases were used. The descriptors used for the search were: “BCG vaccine”, “Diabetes Mellitus type 1” and “Mycobacterium tuberculosis”, with their equivalents in English: “Diabetes Mellitus, Type 1”, “BCG vaccine” and “Mycobacterium tuberculosis”.
Secondly, to form the study sample, the number of new cases of DM-1 in the Basque Country in children under 19 years of age in the period between 2012 and 2022 was selected (that is, an entire decade after withdrawal). of the BSG. The study data was provided by the Department of Public Health and the Department of Health and Quality Information of the Basque Public Health Service (Osakidetza).
The data included, for each year, the total number of new cases by age range (0 to 19 years) and by sex (male, female). As well as the distribution by health areas. Likewise, through the website of the Basque Institute of Statistics ()), epidemiological information was extracted on the quantification of the population under 19 years of age in the Basque Country for each of the years included in the study.
With the study data, a descriptive analysis of the sample was carried out and cumulative incidence calculations were made for each of the years, using the following formula:

The number of cases susceptible to illness was calculated by differentiating in each one-year cohort those exposed and not exposed to BCG according to the year of birth and therefore whether or not they had received BCG in a regulated manner. Finally, for data comparison, the mean was calculated and Student’s T was used as a statistical test with an inference of p: 0.05. SPSS v19.0
Results of the descriptive analysis of the study sample
(Table 1) shows the population data of people residing < 19 years of age in the country. Basque obtained from the Statistical Institute of the Basque Government from the withdrawal of the vaccine in 2012 until a later decade in 2022. The average population of the cohort of those under 19 years of age throughout the decade of study is almost 400,000 individuals for an average population of 2.5 million inhabitants (16% of the population).
| Ages | Year | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
| 0-19 | Total | 384490 | 387782 | 389411 | 393289 | 396028 | 399169 | 400409 | 401766 | 401529 | 399734 | 392372 |
| Men | 197672 | 199388 | 200192 | 202082 | 203625 | 205144 | 205729 | 206528 | 206752 | 206147 | 201586 | |
| Women | 186818 | 188394 | 189219 | 191207 | 192403 | 194025 | 194680 | 195238 | 194777 | 193587 | 190786 |
Table 1. Population in the country Basque during the period studied.
The total number of DM-1 cases debuted throughout the region in this last decade was 663 cases. (Table 2) and (Table 3) shows the data from the descriptive analysis for the period of time between 2013 and 2022.
| YEAR | Number of new cases |
|---|---|
| 2012 | 58 |
| Men | 36 |
| Women | 22 |
| 2013 | 61 |
| Men | 36 |
| Women | 25 |
| 2014 | 52 |
| Men | 28 |
| Women | 24 |
| 2015 | 57 |
| Men | 29 |
| Women | 28 |
| 2016 | 55 |
| Men | 28 |
| Women | 27 |
| 2017 | 56 |
| Men | 28 |
| Women | 28 |
| 2018 | 52 |
| Men | 32 |
| Women | twenty |
| 2019 | 64 |
| Men | 40 |
| Women | 24 |
| 2020 | 56 |
| Men | 28 |
| Women | 28 |
| 2021 | 82 |
| Men | 42 |
| Women | 40 |
| 2022 | 70 |
| Men | 42 |
| Women | 28 |
Table 2. Number of new cases of DM-1 in the pediatric population between 2013-2022 by year and by sex.
| Year | Number of new cases in the population WITH vaccine | Number of new cases in population WITHOUT vaccine |
|---|---|---|
| 2012 | 58 | 0 |
| 2013 | 61 | 0 |
| 2014 | 50 | 2 |
| 2015 | 49 | 6 |
| 2016 | 50 | 5 |
| 2017 | 44 | 12 |
| 2018 | 3. 4 | 18 |
| 2019 | 40 | 24 |
| 2020 | 35 | 21 |
| 2021 | 47 | 35 |
| 2022 | 28 | 42 |
Table 3. Number of new cases of DM-1 in the pediatric population between 2013-2022 according to vaccination status.
Results of cumulative incidence calculations
(Table 4) and (Figure 1) show the cumulative incidence values of DM-1 in the pediatric population under 19 years of age in the Basque Country, in the six years before the withdrawal of the vaccine (2007-2012) and in the ten years after its withdrawal (2013-2022). (Table 5) and (Figure 2) show the cumulative incidence values of DM-1 in the ten years after the vaccine was withdrawn (2013-2022) according to whether or not the pediatric population had received the vaccine. Let us remember that the BCG vaccine was administered compulsorily to all newborns as a single dose at one month of age and, therefore, after its withdrawal, we will find part of the infant population vaccinated and part unvaccinated.
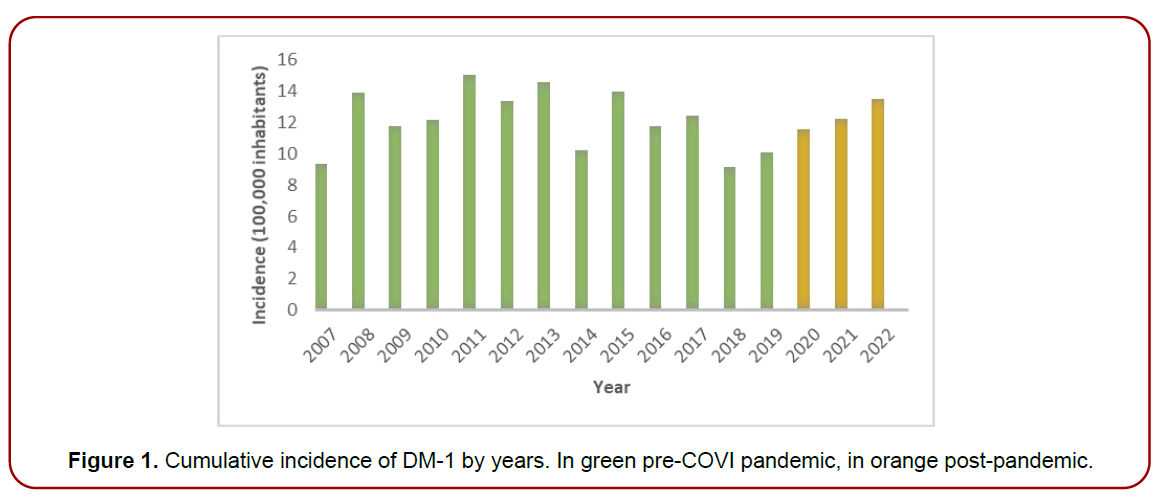
Figure 1. Cumulative incidence of DM-1 by years. In green pre-COVI pandemic, in orange post-pandemic.
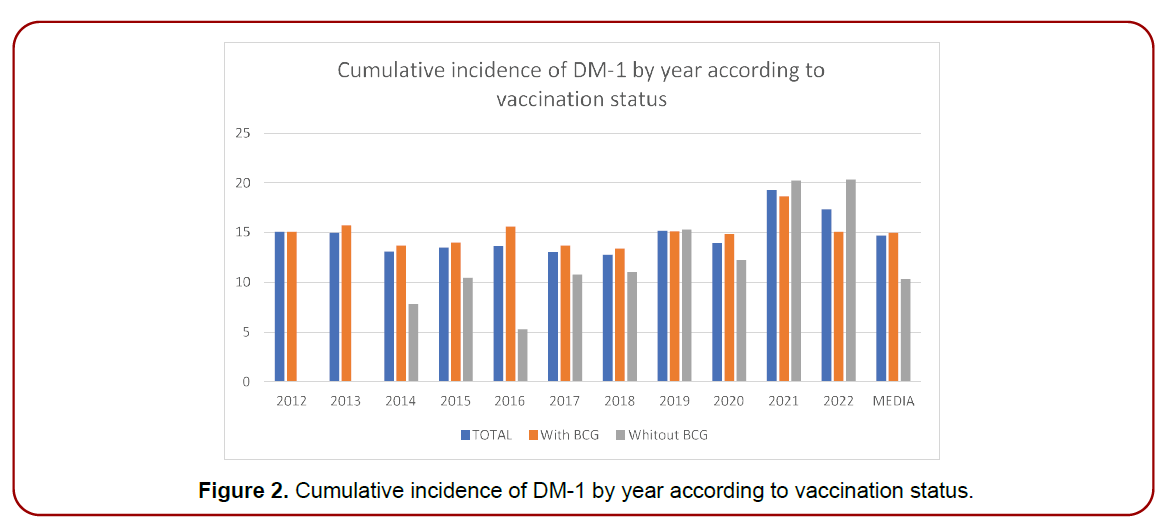
Figure 2. Cumulative incidence of DM-1 by year according to vaccination status.
| YEAR | Cumulative incidence |
|---|---|
| 2007 | 9.36 |
| 2008 | 13.89 |
| 2009 | 11.77 |
| 2010 | 12.19 |
| 2011 | 15.03 |
| 2012 | 13.4 |
| 2013 | 14.59 |
| 2014 | 10.23 |
| 2015 | 13.96 |
| 2016 | 11.8 |
| 2017 | 12.43 |
| 2018 | 9.14 |
| 2019 | 10.12 |
| 2020 | 11.54 |
| 2021 | 12.26 |
| 2022 | 13.5 |
Table 4. Cumulative incidence per 100,000 children of DM-1 per year.
| Year | Global Impact | Incidence with vaccine | Incidence Without Vaccine |
|---|---|---|---|
| 2012 | 15.08 | 15.08 | 0 |
| 2013 | 14.96 | 15.73 | 0 |
| 2014 | 13.1 | 13.68 | 7.79 |
| 2015 | 13.48 | 13.99 | 10.46 |
| 2016 | 13.64 | 15.6 | 5.29 |
| 2017 | 13.03 | 13.7 | 10.79 |
| 2018 | 12.74 | 13.37 | 11.03 |
| 2019 | 15.18 | 15.13 | 15.3 |
| 2020 | 13.95 | 14.85 | 12.24 |
| 2021 | 19.26 | 18.66 | 20.24 |
| 2022 | 17.33 | 15.07 | 20.35 |
| HALF years |
14.7 | 14.99 | 10.32 |
Table 5. Cumulative incidence of DM-1 by year according to vaccination status.
Results of the comparative analysis of accumulated incidents
The average cumulative incidence of DM-1 in the pediatric population under 19 years of age in the six years before the withdrawal of the vaccine was 13,607 cases per 100,000
children. For its part, the average cumulative incidence in the six years after the withdrawal of the vaccine (2013- 2022) was 14.70 cases per 100,000 children, with no statistically significant differences (p:0.23) between both periods. On the other hand, in the period between 2013-2022, after the withdrawal of the vaccine from the schedule, the average cumulative incidence of DM-1 in the vaccinated child population was 14.99 cases per 100,000 children compared to an average cumulative incidence of
10.32 cases per 100,000 unvaccinated children. In this case, no statistically significant differences were found (p:0.08) although there is a tendency to increase cases among the unvaccinated compared to the vaccinated, especially from the cohort of the year after COVID-19.
(Figure 3) shows a linear trend graph to see the evolution of the accumulated incidence by year.
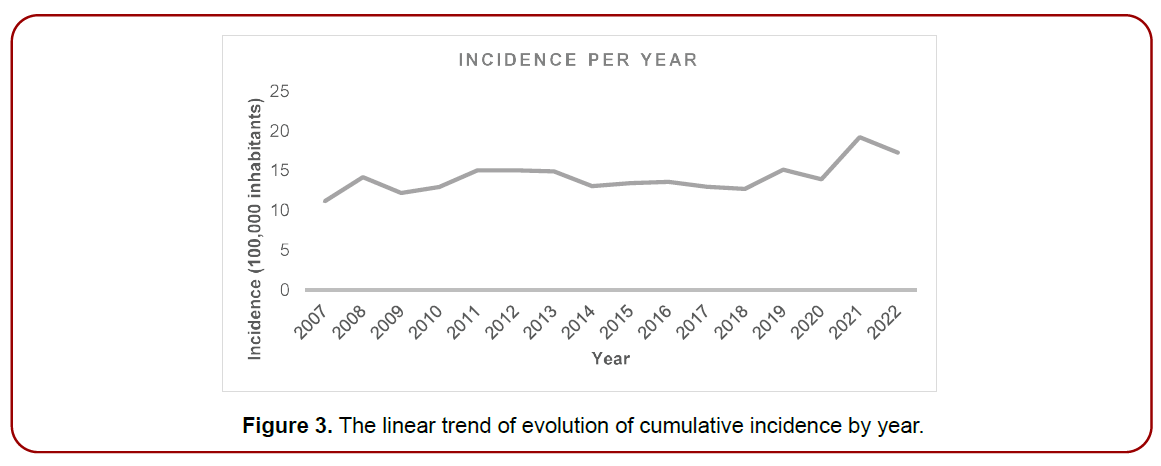
Figure 3. The linear trend of evolution of cumulative incidence by year.
The incidence of cases each year appears quite stable but with an upward trend since 2018.
The BCG vaccine was first synthesized in 1921 from a strain of Mycobacterium bovis. It is a live attenuated vaccine that is used, primarily, to protect against tuberculosis [4]. Studies are becoming more frequent that propose that some vaccines could have heterologous effects or non- specific effects, different from the main use for which they were designed [5]. Thus, for example, the BCG vaccine is used to treat early-stage bladder cancer. Traditionally, it has been considered that the mechanism by which vaccines are capable of generating a lasting immune response is through adaptive immunity. However, there is increasing evidence of the existence of immunological memory in innate immunity. In this sense, it is postulated that every time innate immune cells are exposed to an antigen, they are “trained” for subsequent exposures to said antigen. Therefore, this type of immunological memory is called trained immunity [6].
It has been observed that, thanks to trained immunity, the BCG vaccine could prevent infections caused by pathogens not related to tuberculosis that are responsible for neonatal sepsis or lower airway infections in childhood. Likewise, there are currently two studies in development that seek protection against infection by the new SARS- CoV2 coronavirus with the BCG vaccine. But its use is also being investigated for the treatment or prevention of other infectious processes, but also autoimmune, allergic or oncological processes, although with very different results [7,8].
Currently, the treatment of DM-1 is based on the exogenous administration of insulin. But, as we have previously mentioned, DM-1 is an autoimmune disease, which is why, in the last decade, studies have been carried out on immunotherapy in the treatment of this disease. Thus, it has been discovered that tumor necrosis factor (TNF), one of the most important agents that induce cell death or apoptosis [9], could become a specific immunosuppressive treatment against this disease. This is because the autoimmune T lymphocytes responsible for the destruction of pancreatic beta cells are more sensitive to TNF-induced apoptosis than healthy T lymphocytes. Therefore, TNF would selectively activate cell death or apoptosis. in autoimmune T lymphocytes, sparing healthy T lymphocytes. However, TNF cannot be administered directly into the body due to its high systemic toxicity [10,11].
In tuberculosis infection, the innate immune response plays a very important role as it is the first line of defense against infection. Thus, macrophages have a functional duality: on the one hand, they are responsible for phagocytosing mycobacteria; and, on the other hand, they present the protein antigens resulting from phagocytosis to the T lymphocytes. These lymphocytes are responsible for producing different substances, such as TNF-alpha, which activate the granulomatous inflammatory response to prevent the progression of the infection [12]. Based on the pathophysiological mechanism of tuberculosis infection, we can assume that the BCG vaccine is also capable of stimulating the production of TNF without being toxic to the body. This fact opens the door to the possibility of using BCG against the treatment of DM-1.
In a study carried out by Faustman et al. (2012) demonstrated that the BCG vaccine administered repeatedly in small doses selectively decreases autoimmune T lymphocyte populations and, in addition, they observe an increase in C-peptide levels [13]. In this way, by “eliminating” the lymphocytes responsible for the autoimmune disease, we would be able to improve the damage caused to the pancreatic beta cells and allow their regeneration (hence the increase in C-peptide levels). Likewise, it has been proven that, even in advanced stages of the disease, C-peptide continues to be produced. Therefore, any therapy that allows the regeneration of pancreatic beta cells will be very useful to prevent long-term complications of DM. 1 [14].
However, the selective destruction of autoimmune T cells is not the only mechanism by which the BCG vaccine could be used in the treatment of DM-1. Regulatory T cells play a fundamental role in the control of immunity. In this way, a decrease in regulatory T lymphocytes favors autoimmune diseases and, however, their excess predisposes to oncological processes and infectious diseases [15]. Mycobacterium tuberculosis infection, it has been observed that there is a significant increase in regulatory T lymphocytes to try to control the body’s immune response to stop the infectious process. [16]. Likewise, several studies have shown that after a tuberculosis infection, epigenetic changes occur in the genetic material of some cells to develop a more effective immune response. These epigenetic changes are of various types, such as histone modification, methylation and demethylation of deoxyribonucleic acid (DNA), post-transcriptional modifications or the expression of non-coding ribonucleic acid (RNA) (especially micro-RNA) [17].
It stands to reason that BCG, a vaccine derived from an attenuated strain of Mycobacterium bovis (very close to Mycobacterium tuberculosis), will be able to stimulate the proliferation of regulatory T lymphocytes, as well as produce epigenetic changes in the body’s cells. In this sense, it has been proven that BCG is capable of promoting DNA demethylation processes in the genes that encode regulatory T lymphocytes. This demethylation process not only favors an increase in the number of regulatory T lymphocytes but also favors the synthesis of different micro-RNAs. All of this could stop the autoimmune response responsible for DM-1 [4,17]. On the other hand, another mechanism by which the BCG vaccine could be useful in the treatment of DM-1 is its ability to produce changes in glucose metabolism. In tuberculosis infection, metabolic changes occur in alveolar macrophages as part of the initial potent innate immune response.
One of the most important metabolic modifications is the change of oxidative phosphorylation of glucose by aerobic glycolysis. This change in carbohydrate metabolism appears to limit the survival of Mycobacterium tuberculosis in infected cells [18,19]. In the case of the BCG vaccine, it has been observed that it is also capable of inducing a change in carbohydrate metabolism from oxidative phosphorylation to aerobic glycolysis. This change favors an accelerated use of glucose by cells, which results in a decrease in blood glucose levels and, therefore, in glycosylated hemoglobin (HbA1c). Likewise, it is believed that the reduction achieved in blood glucose levels is comparable to the therapeutic measures currently used for DM-1 (insulin, physical exercise or diet but, in addition, with less risk of generating hypoglycemia in the patients. Patients [4].
However, it should be noted that both epigenetic modifications and the change in glucose metabolism are not observed until 3 years after starting treatment with the BCG vaccine. The reason for this, although it is still unclear, is possibly because in the same way that the autoimmune process develops slowly and progressively, recovery is also slow [4]. The incidence of DM-1 in the Basque Country remains low compared to published population and incidence studies [2], but in our population, there is an upward trend, which is not statistically significant at the moment. This increase in cases could be due to various factors to be considered.
• A real increase in the incidence of cases in the non- BCG exposed cohort vs. the exposed one. As the unexposed population within the cohorts has more population weight, the incidence of cases could
• The effect of a positive bias after the COVID-19 pandemic (7-8), since this infection could have different effects on the development and evolution of autoimmune diseases, including DM-1 itself.
• Population changes experienced in our region in the last 10 years. The immigration of people from other latitudes is a growing fact in Europe and more significantly in various regions of It is known [2,3] that the prevalence of diseases with an autoimmune nature, including DM-1, can be influenced by population changes, associated with the population increase of ethnic groups with a higher prevalence (due to genetic and phenotypic bases) of autoimmune diseases, as is the case of the Maghreb and Latin American populations.
The main conclusion of this study is that the BCG vaccine could have benefits in the control of DM-1 thanks to the selective elimination of the autoimmune T lymphocytes responsible for the disease and, also, the activation of regulatory T lymphocytes through epigenetic changes. Likewise, it promotes changes in carbohydrate metabolism that help control blood glucose levels. Similarly, assessing the heterologous effect of BCG, its use in other autoimmune diseases and even in allergic, infectious and oncological processes could be considered. However, there are still many questions to be clarified. The vast majority of studies developed so far do not establish the number of times it is necessary to vaccinate patients or what dose it should be done. It is also not clear how long it takes for the systemic effect of BCG to be observed in DM-1.
In the specific case of our study, we had several limitations. First, we did not apply any sample size calculation and our study sample may not be representative. Furthermore, this is a study focused on a single Autonomous Community and, therefore, the results may not be extrapolated to other geographical areas. In this sense, as we mentioned previously, DM-1 in our community has a maximum
incidence between 13-14 years of age and a minimum of 0-5 years. In the second part of the study, the children who had not received the vaccine were younger than those who had received it and this could represent a bias when interpreting the data.
Nor did we control, through the data obtained, the ethnic origin of the people affected by DM-1, which could influence the genotypic expression of DM-1 or even having suffered or been exposed to the native BCG itself. For all these reasons, we consider that it is necessary to continue more
studies with a larger number of participants, of different ages, in different geographical areas and with a variable duration of the disease. It is important to know exactly whether BCG could be an effective, safe and long-lasting treatment for a chronic disease as prevalent in childhood as DM-1.
The authors declare that they have not received funding or
have any potential conflicts of interest.