Bala Vaidya1* and Baytieh Lina2
Department of Rehabilitation Medicine, Senior staff specialist, Illawarra and Shoalhaven Local Health District Hospitals, Australia
Medical Co-director, Population and Public Health at Illawarra and Shoalhaven Local Health District Hospitals, Australia
Received: 22 July, 2024; Accepted: 03 August, 2024; Published: 12 August, 2024
Citation: Vaidya, Bala and Baytieh Lina. “Involuntary Emotional Expressive Disorder (IEED) is not to be Confused with Mood Disorder: A Plea to Review Practice.” J Neur Imag Neur Med (2024): 106. DOI: 10.59462/JNINM.2.1.106
Copyright: © 2024 Vaidya B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Involuntary Emotional Expressive Disorder (IEED) is a clinical disorder characterized by uncontrolled and inappropriate laughing or crying episodes; it is a neurological disorder and not just a mood disorder. This disorder is largely not diagnosed correctly or managed appropriately, and this increases the burden on both the patient and carer. IEED is not to be confused with depression bipolar disease or any other mood condition. This disorder occurs following brain impairment events such as stroke, brain trauma, or neural anomalies, or neurodegeneration of isolated and varied etiologies, any of which may set up a scenario of diaschisis, adding to the elusiveness of accurate diagnosis. A sound index of clinical suspicion is required to diagnose IEED. The diagnostic approach requires specific diagnostic tools that incorporate comprehensive historytaking, attentive listening to patients and carers, and targeted assessment tools to help diagnose. Adequate education and diagnostic tools are required to guide the identification of the disorder and fundamental practice.
Emotional instability • Affective disorder • Emotional Expressive Disorder • Affective mood disorder • Post- stroke emotional disorder
Background and Context of Involuntary Expressive Emotional Disorder
Involuntary Expressive Emotional Disorder (IEED) is a clinical disorder characterised by uncontrolled laughing or crying episodes, that is distinct from mood disorders like depression or bipolar disorder and occurs following brain impairment or neurodegeneration of discrete and diverse etiologies [1-6]. The prevalence of IEED varies greatly and is likely misrepresented due to missed or erroneous diagnoses [7-10]. Various studies report estimated rates
of prevalence in certain neurologic and neurodegenerative conditions [7,11], including brain injury, stroke, multiple sclerosis, as well as Alzheimer’s disease, Amyotrophic sclerosis and Parkinson’s disease [2,3,6,7]. Conditions that may prompt the crying variant might include left striatal-capsular infarction; the laughing variant may be brought upon by a subarachnoid hemorrhage. The phenomenon of diaschisis is purported to be at play in various etiologies of either variant or needs clinician suspicion to diagnose [1,9,10,11]. Diagnosis needs to include a series of assessment approaches comprising thorough history-taking with biopsychosocial approaches in the presence of a family member/carer and the use of appropriate assessment tools as the case requires, such as the Centre for Neurologic Emotional Lability scale [4-8].
A selective serotonin reuptake inhibitor (SSRI) is the drug of choice to treat both variants with success to induce reduction of symptoms, increase interaction with friends and family, and return to social activities. An SSRI is effective [11-15] and anecdotally better tolerated than other pharmacological agents. This paper aims to elucidate clinical situations that increase awareness of IEED and instill an index of clinical suspicion within the medical fraternity of IEED as a differential diagnosis in neurological disorders. This serves to aid in the accurate diagnosis and treatment of this burdensome condition. A secondary aim is to provide insight into diagnostic tools and treatment options for IEED to minimize patient and carer burden and improve quality of life.
Towards understanding the barriers: Tackling definitions and terminologies
Sudden, episodic, involuntary, and uncontrolled laughter and crying episodes of pathologic etiology, where the laughter or crying is not triggered by appropriate emotional stimuli or where the expression is exaggerated or contradictory to the emotional valence, occur in neurological disorders that involve the cortico-limbic- sub-thalamic-thalamic-pontocerebellar network either directly or indirectly via diaschisis [1] (Figure 1). Since this is a disorder of emotional expression (affect) and not a disorder of mood or feelings, it has been recently termed “involuntary emotional expressive disorder” [2]. This distinction in the delineation between mood and expression is extremely important as this disorder is often mistaken for a mood disorder rather than a disorder of affect and is consequently improperly treated [3-5]. The many terms used for IEED (such as pathological laughter and crying (PCL), emotionalism, pseudobulbar affect, emotional incontinence, emotional lability, poststroke emotional incontinence (PSEI), including terms listed in King and Reiss’s paper, 201310) and the inconsistency in definitions as well as the many differential diagnoses associated with its manifestations has promoted confusion and uncertainty and has hampered accurate diagnosis of IEED [6]. Furthermore, the need for a thorough investigation into the social impact and the characteristics of everyone has not been adequately appreciated. Listening to patients is of the utmost importance is such cases.
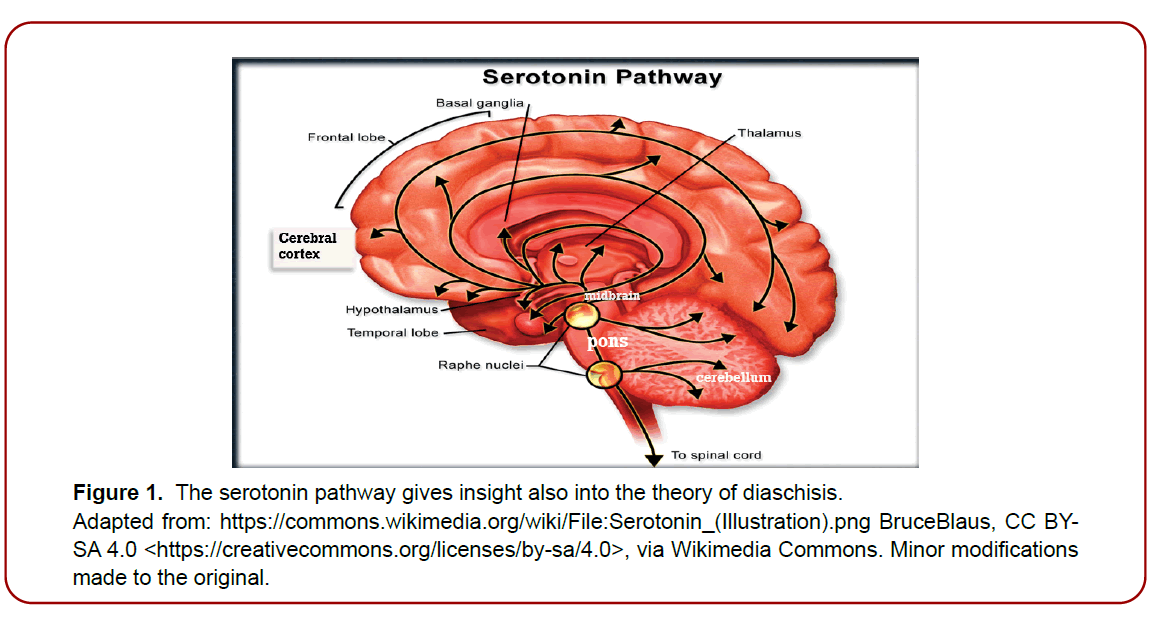
Figure 1. The serotonin pathway gives insight also into the theory of diaschisis.
Adapted from: https://commons.wikimedia.org/wiki/File:Serotonin_(Illustration).png BruceBlaus, CC BYSA
4.0
Involuntary and uncontrolled pathologic laughing and crying states were identified more than a century and a half ago, first described by Darwin in 1872. Wilson, in 1924, postulated that a “Facio respiratory control center” exists in an as-yet unidentified area in the upper brainstem [1] based on Oppenheim and Siemerling’s hypothesis of disinhibition before him in 1886 [8]. The term involuntary emotional expressive disorder was coined by Cummings et al. in 2006 to provide one comprehensive title to replace the multitude of confounding and confusing terms (including emotional incontinence, pathological laughing and crying disorder, and emotional lability) that encompass some of its facets and to offer diagnostic criteria that include “pathologic laughing and crying” and “emotional lability” which have varied symptomatic definitions under the old umbrella term “pseudobulbar affect” [2,3,8,9].
Despite this, accurate diagnosis and appropriate treatment of IEED largely elude clinicians to this day. The need to arrive at an accurate diagnosis of IEED is crucial to attain the best quality of life for patients. The burden of the disorder, at the heart of which is the profound embarrassment to the patient, leads to their social withdrawal and places a heavy load on carers and family members [5,6,10,11,12,13], The impact on individuals with IEED is detrimental and negatively affects participation in rehabilitation programs and community integration and increases the risk of carer burnout. Although useful pharmacological and non-pharmacological measures exist, poor understanding of the disorder, confusion surrounding its etiology and diagnostic parameters and criteria, and the complexity of symptom manifestation within the context of socio-behavioral expectations result in misdiagnosis and under-treatment which prolongs disability and handicap [1,3,6,10,11,12,13].
Mood or affect assessment approaches leading to a diagnosis
Uncontrolled laughter or crying episodes that are not consistent and inappropriate with laughing stimuli are presenting features that need to be distinguished from mood or expression affect before a diagnosis can be made. Assessment tools used for diagnosis of this disorder include the Pathological Laughing and Crying scale and the Centre for Neurologic Study-Lability Scale or CNS-LS [6,7]. The mainstay of pharmacological treatment producing consistent efficacy in the correction of this disorder remains selective serotonin reuptake inhibitors (SSRIs) [12,14,15]. The following examples provide cases in point.
IEED Example 1: IEED Example 1: A 54-year-old male with a history of sudden onset headaches following a strenuous gym session collapsed, prompting an urgent admission to a local tertiary hospital. His diagnosis was a Grade 4 sub-arachnoid hemorrhage with right arterio- venous malformation rupture. He underwent craniotomy and clipping of aneurysms with resection of right frontal arterio-venous malformation. Immediately afterward, he started to exhibit sudden episodes of laughter that had no psychosocial stimuli acceptable to laughter. A thorough assessment was undertaken at the local district hospital Brain Injury Services medical clinic that included an interview and discussion with the patient and his wife. Targeted questions were used to elicit the nature of the symptom presentation and social situation to rule out differential diagnoses such as mood disorders. It was discovered that his outbursts were incongruent to his effect, inconsistent with the setting/situation and unacceptable in the rate of laughter episodes and the duration of episodes. Due to the clinician’s awareness of the possibility of pathological laughing and crying variants within IEED, it led to a strong index of suspicion it could be IEED based on the clinical etiology and the responses to specific questions.
The assessment centered on patient and carer accounts about the social aspects and the embarrassment resulting from the uncontrolled nature of the laughter and the inappropriateness of its trigger. Listening to the patient/ carer was a vital part of the evaluation of the case. The CNS-LS was then used to aid in the diagnosis of IEED. The scale prompts clinicians to think about the questions that would elicit responses that could differentiate between mood and affect. The major symptoms prompting IEED consideration included uncontrollable laughter without any stimulus. According to his wife’s report, he was constantly bursting out into laughter. This resulted in social withdrawal, self-isolation, and inability to meet up with family and friends due to embarrassment. He stopped doing the shopping and driving, which increased the burden on his wife. His wife also reported substantial fatigue due to listening to his laughter all the time.
Given the increased frustration and carer burden, his wife sought help from his general practitioner and was referred to the local Brain Injury Service, where he was initially assessed. The patient’s constant episodes of laughter had no associated emotions, and he reported that he was too humiliated to go out. The CNS-LS was administered, where he scored 19 out of 40, suggesting moderate to severe IEED. A diagnosis of IEED with a pathological laughing variant was made because of the results of the whole part of the assessment.
IEED Example 2: In this example, a 46-year-old female with a background history of transient ischemic attacks, hypertension, and smoking presented with a diagnosis of left striate-capsular infarct resulting in right hemiparesis, expressive dysphasia, dysarthria, and oropharyngeal dysphagia. She was observed to be emotionally labile in rehabilitation, with apparent pseudobulbar involvement. The computed tomography (CT) and magnetic resonance imaging (MRI) results elicited an index of suspicion of IEED. This was based on the clinician’s knowledge of the existence of the condition and clinical suspicion was heightened upon symptom presentation and target investigations to test this differential diagnosis.
Clinical approach and therapy
Example 1: This patient was observed to have developed a generalised tonic-clonic seizure approximately 13 weeks after his emergency craniotomy and clipping of aneurysms and was commenced on levetiracetam. He spent three months in a rehabilitation unit and then discharged home. He received therapy services at home along with neuropsychology follow-up. The patient was later prescribed the SSRI sertraline starting at 25mg daily for the first week, followed by 50mg daily from the second week. Two and a half weeks later, there was an appreciable reduction in his laughter episodes. There was a further progressive reduction in episodes 3 weeks later with the ability to meaningfully interact with his wife and the confidence to go out and shop and meet friends and family once again in social contexts. The degree of resolution of IEED was reflected in the CNS-LS score reduction to 7/40 in 5 weeks. He also showed marked improvement in his mobility and self-care and became independent.
Example 2: This patient exhibited IEED with a crying variant after having sustained a new left striato-capsular infarction on the background of an old striatal infarction causing poor flow in her vertebral and basilar arterial distribution. This was seen on the CT brain. MRI of the brain showed left capsular syndrome involving deep branches of the left middle cerebral artery and left caudate infarct along with right fronto-parietal-occipital infarct which was also old. The patient displayed obvious pseudo-bulbar speech and emotional instability with inappropriate crying. The Functional Independence Measure (FIM) on admission was 67/126. The FIM tool is a validated functional outcome measure used in the rehabilitation setting. It has a physical, functional, cognitive, and social domain section using a linear scale from 1 to 7, with 1 requiring full assistance and 7 being independent. A FIM score of 108 or greater approaches a level of adequate independence at home [16].
The initial dose of the SSRI sertraline was set at 50mg daily on admission to rehabilitation to address the emotional instability and the sporadic spontaneous crying that would occur without any stimuli. This patient responded to sertraline within a week and showed improved participation in the rehabilitation program. She also displayed improved mood and behavior and commenced ambulation with a Quad stick and ankle foot orthosis to her left leg. Her discharge FIM was 83/126. In the first author’s experience, the crying variant within the involuntary emotional expressive disorder scale is the more resilient of the variants, needing a higher SSRI dose and a longer resolution period as was shown in the second example whereby the initial SSRI dose was set at a higher dose (50mg) than in example 1 (25mg).
Mechanisms and impacts of the IEED disorder: Towards understanding and recognition of IEED
IEED is a clinical disorder that is distinct from mood disorders or at least can co-exist with a mood disorder and occurs more often than identified or diagnosed. There is a pressing need to increase awareness of IEED in clinicians assessing patients affected with involuntary, inappropriate laughter and/or crying variants in stroke and traumatic brain injury and in conditions affecting structural brain changes. An increased understanding & recognition of this disorder is necessary to guide logical assessment strategies to distinguish between mood and the clinical, organic disorder of affect that is IEED [1,2,3]. This disorder has a significant impact on quality of life, return to work, and social and personal life integration [3,7]. Social and vocational withdrawal due to embarrassment results in underreported cases. Inaccuracies in the estimation of IEED result from either missing or misdiagnosing the condition due to confusion with mood disorders, co- existence with mood disorders, unfamiliarity with the etiology of the condition and poor understanding of the diagnostic protocol [1,3,10,11,12,13]. The condition has two variants, one being laughter and the other crying, which manifest on a spectrum dictated by duration, intensity, context, behavior, and limits of control [3,4]. The term IEED covers the spectrum from emotional lability whereby there is some level of control of emotional expression and the expression itself may be congruent to the situation but exaggerated. PLC is on the spectrum where laughter or crying is incongruent to the situation, and the valence is uncontrollable and has an inapt duration [3,4,17]. Strowd et al. state that these different manifestations of the condition may indicate a greater degree of pathology and, therefore, more disinhibition [11].
The two example cases outlined in this paper exhibited either variant with classic presentations where the laughter or crying was not triggered by any stimuli, the frequency was exaggerated, and the duration was out of the expected norm. Questions had to be targeted towards the inhibition of emotions and their regulation. The use of the CNS-LS, a validated screening tool, helped focus the questions on factors that trigger the episodes, the frequency, duration and ability to control these episodes, the incongruence of the expression to actual mood and other factors that distinguish it from mood disorders [6,8]. A cut-off of 17 is regarded as indicative of disease, although Strowd et al. argue that using 13 as a cut-off in certain presentations may be more useful [11]. Alternative tools that guide IEED diagnosis include the Pathological Laughing and Crying Scale and the Affective Lability Scale [5,6,8,11].
Recognizing the distinction between mood and affect is at the heart of a true diagnosis of IEED, and these scales have questions that teach the difference.6 King et al. (2013) [10] depict the phenomenon as an “uncoupling” of the experience of emotion that is subjective (mood) from the responses to emotions that are objective (affect) where motor and autonomic reactions are involved. Some have described the effect of this uncoupling as disinhibition or dysregulation of emotional expression because of disruption of the cerebro-pontocerebellar neurocircuitry and its neurochemical network [6,7].
Neuroimaging has been pivotal in underpinning the relevant area and the network system of the pathological variant of laughter and crying. Using neuroimaging exploration Klingbeil et al (2021) [1] implicate the “Cortico- Limbic-Subcortical-Thalamo-Ponto-Cerebellar Network” in the disorder as per the theoretical framework put forward by King and Reiss 2013 [10], where the pathophysiology of the disorder develops from the disruption of this network involved in emotional expression and regulation, with resultant disturbance of the diffuse neurotransmitter system [1]. At the heart of this network is the notion of diaschisis. The theory of diaschisis describes conditions whereby damage or changes in the surrounding structures have an indirect influence on this network and effectively disrupt serotonergic and possibly another type of neurotransmission [1]. This is probably the reason that consistent efficacy is seen in the use of SSRIs [12,14,15]. (Figure 1) gives insight into how the idea of diaschisis works where such a disruption can induce affective behavior.
The index of clinical suspicion must be high for such cases where etiology is unclear, but diaschisis may be an active process. The clinician needs to first understand the nature of the anomalies of laughter or crying episodes. A thorough investigation is required in the presence of a family member or other significant person who lives with the affected patient. The tools, the CNS- Liability Scale, the PLC scale, etc. can themselves teach and guide clinicians in this delineation. It is of the utmost importance that the clinician listens to the patient care and others who are involved in comprehending the nature of the symptoms to come to an accurate diagnosis. Neuroimaging, pharmacological effects, and anomalies in presentations of IEED support the idea of disruption of neurotransmission at the focal site for these motor behaviors by modulation of the neurotransmitters involved. It appears that each variant of IEED is due to disruption of a focal region that is particular to the motor expression. These regions are unclear to date.
The main neurotransmitter implicated is serotonin, where modification of the presynaptic serotonin transporter occurs within serotonergic pathways ascending from the raphe nuclei all the way to the frontal cortex, serving to reduce circulating serotonin [12,15,17]. (Figure 1) depicts the possible scenario. Pathological crying is a result of lower binding ratios of these transporters in the midbrain and pons [9,10,12]. The efficacy of SSRIs in reducing IEED symptoms support this theory. The increased availability of serotonin saturates the postsynaptic receptors through binding. This then favorably modulates the corticolimbic and cerebellar pathways and deters pathologic laughing [15]. In addition to serotonin, other neurotransmitters may be involved either in isolation or in synergy. These include glutamate, dopamine, acetylcholine, norepinephrine, and corticosteroids [3]. However, it seems serotonin and glutamine may be the main neurotransmitters of importance as this is supported by studies that have found that the anti-glutamatergic modulators dextromethorphan/ quinidine work in certain cases of IEED presentations [3,11].
Dextromethorphan is a sigma-1 receptor agonist and, although it may have a role in the modulation of the serotonergic system, [3] its effect on glutamatergic neurotransmission through action on the sigma-1 receptors is likely of particular importance [3]. It is worth mentioning that patients who are refractive to SSRIs are given selective adrenergic receptor inhibitors and dopaminergic agents as second-line treatment with good effects [12]. The effectiveness of SSRIs in attenuating the frequency and severity of IEED manifestations has been supported by Grade 1 studies, including a Cochrane review and five randomized controlled trials (RCTs). [12,14] In 2 RCTs, tricyclic antidepressants were effective. However, the first author of the current paper, in keeping with Kim (2016,2017) and others [8,12,13,14,15] recommend the use of SSRIs as the first line option in IEED treatment as SSRIs are more effective, act faster than tricyclic antidepressants, and confer considerably better tolerance. Parvizi et al. (2006) report on how discontinuation of medication other than SSRIs (such as dextromethorphan/quinidine) occurs due to intolerance to side effects.
This could also be because SSRIs confer appreciable effects in reducing IEED symptoms within days of commencement at much lower dosages than those prescribed for mood disorders, serving to minimize side effects with their use.8 Dosage titer may be where optimization of the effectiveness of these agents lies and stepwise increases in dosing. More research in this area is warranted. In the examples of the patients given SSRIs, they work effectively and promptly with excellent tolerance; reduction in symptoms has been seen in stepwise increases in dosages. A gradual but notable reduction in symptoms was seen with the commencement of SSRIs, with near resolution of symptoms at one-week post- treatment for the crying variant and at two weeks post for the laughter variant. The first author of this paper observes anecdotally that the crying variant appears to respond quicker to SSRIs than the laughter variant. The patient with the laughing variant had scored 19/40 on the CNS- LS pre- SSRI commencement, suggesting a moderate to severe affective disorder, then scored 7/40 approximately 5 weeks post, implying a move towards normalcy. In response, there was increased interaction with friends and family and a return to social activities in both cases. Morland et al. (2015) present a case study using brain SPECT showing how pathological laughter could be a factor of diaschisis affecting serotonergic neurotransmission given that their patient was successfully treated with the SSRI fluoxetine at a dose of only 5mg/day. 18 This patient was a 40-year-old man who sustained a pontine stroke, but the MRI showed no lesion in the cerebellar-pontine region or surrounding structures. Brain SPECT perfusion imaging, however, did show marked hypoperfusion in the right frontal and temporal-insular regions originating from the pontine, supporting a diaschisis situation due to the pontine lesions and showcasing the possible pathway of laughter regulation. This concept is illustrated in (Figure 1) [18].
Further to this hypothesis are rare presentations documented with unusual cases of inappropriate, uncontrolled laughter or crying of limited duration due to inhalation or alternate administration of a particular substance or substances. Zellers et al. (1990) present a case whereby a man inhaled an insecticide and started laughing inappropriately and uncontrollably a short time afterward. The laughter continued for one hour and 40 minutes [19]. A case of chemotherapy administration of a regimen consisting of carboplatin, docetaxel, and trastuzumab provoked unstimulated and uncontrolled episodes of laughter interjected with bouts of crying in a 52-year-old woman [20]. The episodes began on day 2 after chemotherapy treatment, peaked at about day 3 and 4 and completely resolved by day 6 post-treatment. Each episode lasted approximately 5-10 minutes with a frequency of 4 episodes a day.
There are idiopathic cases of transient laughter or crying that do not fit into the definition outlined by IEED and do not align with similar nosology. Of note is that the treatment for these conditions was not SSRIs but nonstandard treatment, such as diazepam in the first case, was used with good effect. 19 Further explorations into such anomalies are required. The multitude of etiologies of IEED, the many varied presentations of the condition and the targeted treatment options and treatment approaches need to be disseminated to the medical community at large so that appropriate treatment can reduce patient and carer burden. An index of clinical suspicion must be cultivated in a wide scope of patient populations to include anomalies.
The addition of IEED to the medical curriculum is justified. The development of assessment and diagnostic tools for IEED is warranted. There have been too manymisdiagnoses related to this disorder which continues to this day in this clinical space, increasing patient and carer burden and suffering, in addition to misdirection of resources. There remains the pressing need to instill a targeted index of clinical suspicion in students and clinicians alike for this condition as its accurate diagnosis and treatment continue to elude us a century after the condition was elucidated.
Health professionals need to appreciate that IEED is a condition of disordered affect rather than mood. IEED is not to be confused with depression bipolar disease or any other mood condition. The condition of IEED, its etiology, pathophysiology, diagnosis, and treatment need to be taught widely in medical education so that it instills a high index of suspicion in clinical practice. The diagnostic criteria and protocol for IEED needs to be unanimously established and its treatment approaches well delineated.