Marwan El-Deyarbi1,2, Luai Ahmed3, Jeffrey King4,5, Ahmed Al Juboori6, Nirmin Mansour6 and Salahdein Aburuz1*
Department of Pharmacology, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, UAE.
Department of Pharmacy, Oud Al-Touba Diagnostic and Screening Clinic, Ambulatory Health Services, Abu Dhabi Health Services Co. (SEHA), Al Ain, UAE.
Institute of Public Health, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, UAE.
David Geffen School of Medicine at the University of California, Los Angeles, CA USA.
Department of Veterans Affairs, Greater Los Angeles, Department of Geriatrics and Extended Care, Los Angeles, CA USA.
Division of Endocrinology, Oud Al-Touba Diagnostic and Screening Clinic, Ambulatory Health Services, Abu Dhabi Health Services Co. (SEHA), Al Ain, UAE
Received: 08 March 2024; Processed: 30 May 2024; Accepted: 11 June 2024
Citation: Aburuz, Salahdein. “Metabolic and Renal Outcomes of Empagliflozin among Emirati Patients with Type 2 Diabetes.”J Pharmacol Drug Deliv (2024): 104. DOI: 10.59462/JPDD.2.1.104.
Copyright: © 2024 Aburuz S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: In the last few decades, diabetes mellitus has become one the most prevalent disease and the 7th leading cause of death worldwide. Many studies shows that empagliflozin improves glycemic control in type 2 diabetic (T2DM) patients and added to several clinical treatment pathways, however, metabolic and kidney outcomes studies have not been stablished on United Arab Emirates (UAE) national patients.
Objective: To examine the metabolic outcome of empagliflozin when added as an antidiabetic drug for Type 2 diabetic patients and the short-term effect on renal outcomes.
Methods: A retrospective chart review, recruiting 188 patients in 2 groups of uncontrolled T2DM patients with glycosylated hemoglobin (HbA1c) ≥ 7.5%. A group receiving either empagliflozin 10 or 25mg tablet as a newly added glucose-lowering agent and a group receiving standard of care (SOC). For both groups, other diabetic medication remains unchanged for at least the first 3 months, with a median follow-up time of 1-year in Oud Al Touba Diagnostic Center in Al Ain, UAE.
Results: The effect of empagliflozin 10mg on HbA1c reduction was insignificant compared to SOC with mean HbA1c reduction of 0.3 and 0.4 compared to baseline data at week 12 and 48 respectively, however, patients who started or shifted to empagliflozin 25mg had a significant difference in the mean change from the baseline to study exit compared to SOC of 0.5%, 95% CI 0.36 ‒ 0.78, P < 0.05), with mean HbA1c reduction of 0.5 and 0.6 at week 12 and 48 respectively, compared to baseline data. Empagliflozin-treated patients reported a transient reduction of estimated glomerular filtration rate (eGFR) from baseline in both empagliflozin groups (mean [±SD] changes of -1.5 ± 2.7 and -2.9 ± 5.3 in empagliflozin 10 mg and 25 mg groups respectively.)
However, eGFR levels at week 48 have been improved in both empagliflozin groups with a mean increase at the end of the study of 2.2 mL/ min/1.73m2 in the pooled empagliflozin group compared to baseline with no reported kidney injury events. Uncomplicated urinary tract infection was reported more frequently (0.9%) with empagliflozin 25 mg group.
Conclusion: Among patients receiving empagliflozin therapy, those in the empagliflozin 25 mg group had a significant improvement in metabolic parameters, particularly in patients with HbA1c ≥ 9%, with more frequent urinary tract infections.
T2DM • SGLT2 • HbA1c . eGFR • UTI • LDL-C • Genital infection.
Type 2 diabetes mellitus has become the more prevalent type of diabetes in many countries and is characterized by progressive insulin deficiency and resistance [1,2] and contributes annually for 5.2 million deaths worldwide [3]. Diabetes mellitus disease risk and progression are influenced by various risk factors either genetic or environmental, both collaboratively can intensify the loss of pancreatic beta cells and modify its function that diagnosed clinically by hyperglycemia [1]. Chronic hyperglycemia is the major risk factor for many macrovascular as well as microvascular disease in type 2 diabetes [4] and explains the increased mortality rate of the disease [5,6].
Several glucose-lowering agents or intensive glucoselowering strategies have been shown to reduce the chronic hyperglycemia after a prolonged follow-up period in patients with T2DM, however, may be associated with adverse cardiorenal events [7]. Empagliflozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, optimizes glucose control through decreasing renal glucose reabsorption and increasing urine glucose excretion [8,9] and shows a significant reduction in glycosylated hemoglobin as mono- or add-on therapy in type 2 diabetic patient with chronic kidney disease [10- 13]. Thereby, SGLT2 medication has been approved to improve glycemic control in patients with type 2 diabetes [14,15] and added to evidence-based guidelines and clinical pathways in treating type 2 diabetes mellitus [16].
Evidence in reducing serious renal events and hospitalization from cardiac disease has been achieved with antihyperglycemic agents such as SGLT2 inhibitor drugs, regardless the presence or absence of diabetes and not seen in agents with higher antihyperglycemic effects [17]. These observations were consistent with empagliflozin in the EMPA-REG OUTCOME trial [18], as well as, in the EMPEROR-Reduced trial [19] and can be explained by the osmotic diuresis and natriuresis caused by the selective SGLT2 inhibition of empagliflozin, leading to intravascular volume contraction and hemoconcentration [20,21]. Furthermore, empagliflozin has favorable effect on weight loss and in blood pressure reductions, vascular resistance and arterial stiffness without increasing heart rate [11,22,23].
Several studies have demonstrated the efficacy of empagliflozin on HbA1c as add-on therapy for type 2 diabetic patients and provide additional benefits such as blood pressure reduction and weight loss beyond the glucose-lowering effect [24]. Besides, empagliflozin reduces all-cause healthcare costs and resource utilization when initiated in T2DM patients [25]. Ramos and his colleagues had witnessed this by showing empagliflozin treatment is cost-effective and contributes to additional Quality Life years compared to other drug classes such as dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists with standard of care [26].
However, U.S. Food and Drug Administration (FDA) rises the concern that symptomatic hypotension when initiating empagliflozin in the elderly, patients with renal impairment and in patients with low systolic blood pressure or on diuretics may be caused due to contraction of plasma volume [27]. Moreover, the FDA has revised labels of SGLT2 inhibitors for diabetes and warned from serious conditions that can result in hospitalization, due to the increased risk of ketoacidosis, urinary tract infections and the rare occurrences of Fournier’s gangrene, a serious genital infection, from the normal use of such agents including empagliflozin [15,27]. In addition, several studies show that empagliflozin is associated with increased levels of cholesterol low- and high-density lipoprotein [12,13]. Therefore, healthcare professionals should take into consideration those unpreventable clinical effects while prescribing and monitoring empagliflozin therapy.
In this study, we examined the metabolic effects and the cardiorenal outcomes of empagliflozin, as an addon therapy, in a population of Emirati patients with type 2 diabetes and if additional prescribing or monitoring guidelines are warranted.
Study design
In this retrospective chart review study using the patient’s electronic medical record (Cerner health information), we recruited 188 type 2 diabetic patients in two groups of, an empagliflozin group were empagliflozin added on the top of the diabetic regimen and a control group receiving standard of care but not on empagliflozin tablets. Both groups have similar baseline metabolic and renal characteristics and matched our inclusion criteria. For both groups, other diabetic medication remains unchanged for the first 3 months but can be intensified or changed after 3 months according to local guidelines.
Cerner has been used to record the initial assessment data of clinical findings, baseline data of the recruited population and the evaluation data during the follow-up treatment after the initiation of empagliflozin. All recruited patients have median follow-up time of 1-year with the endocrine or chronic disease clinics in Oud Al Touba center in Al Ain, UAE.
Study outcomes
The primary outcomes were adjusted mean reduction in HbA1c within 12 months compared to the control group during study period and incident or worsening nephropathy measured by adjusted mean (SE) difference versus SOC in change from baseline in eGFR. Secondary outcomes included the effect on low-density lipoprotein (LDL-C) levels compared to baseline. The study also assessed the safe use of empagliflozin based on the reported adverse events during treatment or reported within 7 days after the last dose of empagliflozin and any recorded thromboembolic, urinary tract infection (UTI), genital mycotic infection, volume depletion and metabolic ketoacidosis events.
Statistical Analysis
Data was analyzed blindly using IBM SPSS (version 26) statistics software and included the comparison of the 10mg dose and 25mg dose of empagliflozin versus control through repeated measures analysis for the changes of HbA1c, eGFR and LDL from baseline by Student’s t-test. Statistical significance used only as a guide towards determining the statistical significance of the treatment in patient’s results. We analyzed the frequency of occurrence for the reported adverse events, such as urinary tract infection and genital infections using Chi-Square test to indicate if series of events occurred with equal frequency among the recruited patients. The calculated sample size of study was 78 in each group, using the GPower® software (version 3.1.9.4), with a power = 80%, effect size =0.4 and the ratio of sample size of both groups = 1 with population standard deviation (SD) =1.
Study participants
In our study, we recruited 188 patients who matched our inclusion criteria. A total of 111 diabetic patients were initiated on empagliflozin (54 patients on empagliflozin 10mg and 57 patients on empagliflozin 25mg) and 77 patients were receiving SOC for diabetes management. At baseline, the pooled empagliflozin group and SOC group have balanced demographic and clinical characteristics (Table 1).
| Characteristics | Empagliflozin 10 mg | Empagliflozin 25 mg | Control (SOC) (n-77) | P Value |
|---|---|---|---|---|
| Demographic | ||||
| Age-Yr | 59.3 ± 12.67 | 58.1 ± 12.7 | 55 ± 12.6 | 0.27 |
| Female | 41 (76) | 37 (65) | 57 (74) | 0.57 |
| Weight - kg | 75.5 ± 15.7 | 79.2 ± 17.5 | 76.4 ± 21.4 | 0.11 |
| Clinical | ||||
| HbA1c - % | 8.6 ± 1.01 | 9.2 ± 1.04 | 8.8 ± 0.92 | 0.34 |
| eGFR | 99.5 ± 20.7 | 94.9 ± 22.3 | 95.1 ± 21.7 | 0.94 |
| LDL-C | 2.3 ± 0.89 | 2.5 ± 0.78 | 2.4 ± 0.77 | 0.57 |
| SBP - mmHg | 139.4 ± 16.2 | 138.8 ± 17.1 | 138.6 ± 16.8 | 0.15 |
| DBP - mmHg | 82.9 ± 9.6 | 84.4 ± 10.2 | 83.5 ± 9.8 | 0.08 |
| Medication | ||||
| Insulin | 5 (9) | 8 (14) | 13 (17) | - |
| Antihypertensives | 39 (72) | 48 (84) | 60 (77) | - |
| Diuretics | 9 (17) | 11 (19) | 22 (28) | - |
| Lipid-lowering agent | 46 (85) | 52 (91) | 61 (79) | - |
| Medical History, 1 year preceding the study | ||||
| Recurrent UTI | 0 (0) | 3 (5) | 0 (0) | - |
| Genital Infection | 0 (0) | 0 (0) | 0 (0) | - |
| MI /Stroke | 0 (0) | 0 (0) | 0 (0) | - |
| Prescriber privilege | ||||
| Specialist / Consultant | 41 (76) | 49 (86) | 36 (47) | - |
| GP Practitioner | 13 (24) | 8 (14) | 41 (53) | - |
Table 1. Baseline characteristics of study participants.
The mean age in the 10mg and 25mg groups were 59.3 and 58.1 years and they were 76% and 65% female respectively. The mean Hb1Ac at baseline was 8.6 percent in the 10mg group and 9.2 percent in the 25mg group and eGFR levels were 99.5 and 94.9 mL/min/1.73m2 respectively. In the control group, the mean age of patients were 55 years and 74% were females, with mean Hb1Ac of 8.8% and eGFR level of 95.1 mL/min/1.73m2 (Table 1).
Glycemic control
During study period, 21 patients of the empagliflozin group (30%) started and continued with empagliflozin 10 mg, with mean (±SD) HbA1c 8.3 ± 0.9 on week 12 and 8.2 ± 0.8 on week 48. The dose escalated from 10mg to 25mg in 33 patients (61%) from the empagliflozin 10mg group after 3 months. HbA1c was not measured in 6 patients (18%) before dose switching with limited mean HbA1c difference of -0.1%. In our study, 57 patients initiated on empagliflozin 25mg with mean (±SD) HbA1c 9.2 ± 1.04 and at the end of study period patients who started or switched to 25mg have a mean (±SD) HbA1c of 8.5 ± 1.02 (Table 2).
| Variables | 10mg | 25mg | Combined | Control (SOC) |
|---|---|---|---|---|
| Week 12 | n=54 | n=57 | n=111 | n=77 |
| HbA1c - % | 8.3 ± 0.9 | 8.7 ± 1.1 | 8.5 ± 0.8 | 8.6 ± 0.86 |
| eGFR - ml/min/1.73 m 2 | 98 ± 21.4 | 92 ± 21.8 | 95 ± 21.1 | 95.7 ± 21 |
| LDL-C - mmol/l | 2.4 ± 0.87 | 2.6 ± 0.84 | 2.4 ± 0.85 | 2.4 ± 0.85 |
| Week 48 | (n = 21) | (n = 90) | (n = 111) | (n = 77) |
| HbA1c - % | 8.2 ± 0.8 | 8.6 ± 1.02 | 8.4 ± 1.1 | 8.7 ± 0.84 |
| eGFR - ml/min/1.73 m 2 | 99.7 ± 21.6 | 95.2 ± 20.4 | 97.5 ± 21 | 95.3 ± 20.6 |
| LDL-C - mmol/l | 2.5 ± 0.86 | 2.8 ± 0.87 | 2.7 ± 0.87 | 2.5 ± 0.85 |
| Weight - kg | 75.1 ± 13.4 | 77.4 ± 16.2 | 76.3 ± 14.7 | 76.9 ± 18.6 |
| SBP - mmHg | 137.3 ± 15.1 | 135.6 ± 16.9 | 136.5 ±16.1 | 137.5 ± 16.7 |
| Adverse events | ||||
| Recurrent UTI | 0 (0) | 1 (1.7) | 1 (1.2) | 0 (0) |
| Genital Infection | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MI /Stroke | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Table 2. Follow-up data at 12 and 48 weeks.
At 12 weeks, the mean differences in HbA1c in the empagliflozin 10mg or 25mg groups compared baseline data were -0.3% and -0.5% respectively and the pooled empagliflozin group has mean differences in HbA1c of -0.4 at study exit compared to baseline readings (Figure 1). After 48 weeks, 21 patients only continued empagliflozin 10mg achieving -0.4 mean difference in HbA1c compared to recruitment data and at study exit for those patients who received at least one dose of empagliflozin 25mg had mean differences in HbA1c of -0.6 % compared to baseline data with a significant difference in the mean change from the baseline to study exit compared to control group (SOC) of 0.5%, 95% CI 0.36 ‒ 0.78, P < 0.05) (Table 3).
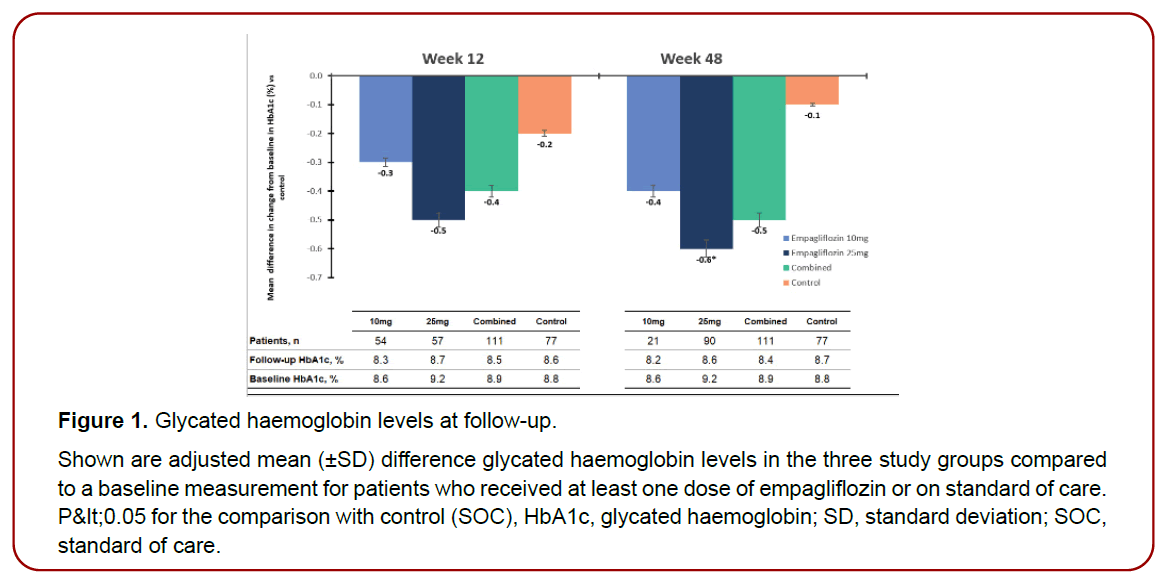
Figure 1. Glycated haemoglobin levels at follow-up.
Shown are adjusted mean (±SD) difference glycated haemoglobin levels in the three study groups compared
to a baseline measurement for patients who received at least one dose of empagliflozin or on standard of care.
P<0.05 for the comparison with control (SOC), HbA1c, glycated haemoglobin; SD, standard deviation; SOC,
standard of care.
| Follow-up data | Baseline | Study exit | Mean difference | |||
|---|---|---|---|---|---|---|
| Empagliflozin | Control | Empagliflozin | Control | (95% CI) | P value | |
| Empagliflozin10mg | 8.6 ± 1.01 | 8.8 ± 0.92 | 8.2 ± 0.8 | 8.7± 0.84 | 0.3(0.28- 0.43) | 0.23 |
| Empagliflozin25mg | 9.2 ± 1.04 | 8.8 ± 0.92 | 8.6 ± 1.02 | 8.7± 0.84 | 0.5 (0.36 -0.78) | 0.04* |
| Empagliflozin 10 + 25mg | 8.5 ± 0.94 | 8.8 ± 0.92 | 8.4 ± 1.1 | 8.7± 0.84 | 0.02 (0.12-0.55) | 0.98 |
Table 3. Empagliflozin follow-up data.
Furthermore, for all patients who received empagliflozin, they achieved a mean reduction of 0.5% at study exit compared to baseline readings (Figure 1), however the difference in the mean change from the baseline to study exit compared to SOC did not reach statistical significance 0.02%, 95% CI 0.12 ‒ 0.55, P = 0.98) (Table 3).
Renal outcomes
In the empagliflozin group, eGFR level was measured only in 91 patients (82%) before empagliflozin initiating and lab test ordered only for six patients (18%) before dose escalation of empagliflozin from 10mg to 25mg at 12 weeks, however, eGFR levels were measured at least 2 times during the follow-up period. At week 12, a transient reduction of eGFR levels has been observed in both empagliflozin groups (mean [±SD] changes from baseline, -1.5 ± 2.7 in the group receiving empagliflozin 10 mg and -2.9 ± 5.3 in the group receiving 25 empagliflozin)
However, eGFR levels at week 48 have been improved in both empagliflozin groups with mean difference of the pooled empagliflozin group of 2.2 mL/min/1.73m2 compared to baseline. In the empagliflozin group, the mean eGFR increase from baseline in the empagliflozin 10mg group was 0.2 mL/min/1.73m2 and in the empagliflozin 25mg group was 0.3 mL/min/1.73m2 (P > 0.05 for both comparisons with baseline).
Cardiovascular risk factors
Through the whole of the study, patients in the empagliflozin group, as compared with standard of care, were associated with lower values for weight at the end of the study period with mean (±SD) changes from baseline of -1.1 ± 3.5 in the patients receiving empagliflozin and 0.5 ± 2.7 in the SOC group. For both empagliflozin groups, there were small reduction in mean systolic blood pressure from baseline at week 48 (mean [±SD] reduction in 10mg empagliflozin group from baseline of -2.1 ± 5.4 and in 25mg empagliflozin group of -3.2 ± 5.8, compared to -1.1 ± 5.2 in SOC group) and small increase in LDL-C levels by 0.3 mmol/L in the pooled empagliflozin group and 0.1 mmol/L only in the control group at week 48, compared to week 12, however both results were insignificant.
Safety and adverse events
Furthermore, 13 patients (16.6%) were on diuretics before starting empagliflozin without dose adjustment. No new cases of UTI or genital infections have been reported at the end of the study, however, at baseline 3 females (2.7%) in the empagliflozin group reported recurrent UTI before drug initiation and from those patients 1 patient (0.9%) in the empagliflozin 25mg group continued reporting UTI during study period. No cardiovascular events, volume depletion or metabolic ketoacidosis events have been reported during the follow-up period in both study groups.
Empagliflozin is a novel drug molecule, indicated in the treatment of type 2 diabetes mellitus to improve glycemic control in adults [14]. The recommended starting dose is of Empagliflozin is 10 mg once daily either as monotherapy or add-on combination therapy with other diabetic medication [28], however, limited studies recommend the best initiation dosing regimen based on HbA1c levels. During recruitment, 54 patients started with 10 mg empagliflozin tablets and 21 (39%) patients have continued the same dose for an average of 3 months only, before increasing the dose to empagliflozin 25 mg in 33 patients. HbA1c was not measured in 6 patients before dose switching to indicate the efficacy of the medication or the need for dose escalation, however, initiation of 10 mg dose in those patients was possibly to measure the tolerability of the medication before prescribing the planned 25 mg dose of empagliflozin.
At the end of follow-up period, 21 patients (27%) in the empagliflozin 10 group achieved -0.1 percentage adjusted mean differences in HbA1c compared to baseline and SOC. This result can be due to the relatively low HbA1c level upon recruitment or the need for a higher dose of empagliflozin, which requires further elucidation in a larger study sample. The effect of empagliflozin on HbA1c reduction was consistent when shifting from empagliflozin 10 mg to 25 mg groups. However, the patients who started or switched to empagliflozin 25mg shows -0.5 percentage adjusted mean differences in HbA1c compared to baseline and -0.3 percentage to SOC, with an overall HbA1c adjusted mean reduction for the combined groups of -0.2 compares to SOC. In both groups neither sex nor age impacted the adjusted HbA1c significantly, which is consistent with other studies [9].
Empagliflozin is a reversible and 5,000 times more selective for SGLT2 versus SGLT1, the major glucose transporter in the gut. SGLT2 is highly expressed in both kidneys, compared to other tissues where expression is very low or absent. It’s the predominant transporter for glucose from the glomerular filtrate back into the circulation and is dependent upon the GFR and blood glucose concentration [29]. Assessment of renal function is recommended at empagliflozin therapy initiation and periodically (at least yearly) during treatment [28]. In our study to quantify the effect of empagliflozin exposure on renal function, assessment of eGFR was measured at the beginning of group, at the end of the study follow-up and as possible before dose escalation. Despite the estimated mean difference of eGFR parameter for empagliflozin 10 mg and 25 mg groups at the end of follow-up period from baseline were generally comparable to SOC, both results were statistically insignificant. The diversity in the eGFR outcomes, can be further investigated with other diabetic variables in a regression model or subgroup analysis.
Some differences were evident during therapy initiation with transient reduction of eGFR and with mean difference of -1.2 mL/min/1.73m2 in the first 3 months compared to SOC, however eGFR levels have been improved at the end of the follow-up period compared to SOC, which is in consistent with previous studies [30,31]. In placebocontrolled trials and post-marketing studies the incidence of UTI, genital infections and the life-threatening cases of diabetic ketoacidosis were higher in patients treated with empagliflozin, moreover, the overall frequency of UTI reported as adverse event was higher in patients treated with Empagliflozin 10 mg than Empagliflozin 25 mg, which was not evidence in our study may be because of the small study sample [32,33].
The overall frequency of urinary tract infection (including pyelonephritis or urosepsis) nor genital infections was not significant during follow-up period in both empagliflozin groups, however, 3 cases in the female group reported recurrent UTI infection before study initiation and 1 of these 3 patients continued to have UTI during empagliflozin treatment. Regular follow-up may be required for those patients and temporary interruption of empagliflozin may be considered in patients developing complicated urinary tract infections [34]. In addition, initiation of empagliflozin increases excretion of sodium resulting in osmotic diuresis and reduced intravascular volume [35,36]. During our study no cases of volume depletion (including dehydration and decreased ambulatory blood pressure) or ketoacidosis have been reported in both groups, especially in our elderly participants.
Although in our study we can’t prove the cardioprotective effect of empagliflozin as evidenced by many studies [37], study participants didn’t report any myocardial infarction or stroke during the study period. Besides, the study shows empagliflozin’s safety profile when added to other diabetic medication with no adverse reactions or drug-drug interactions reported, however, additional drug interaction studies should be performed.
The addition of empagliflozin medication led to improvements in many clinical and metabolic parameters in our Emirati patients with type 2 diabetes, particularly in patients with HbA1c ≥9%, with no reported serious renal or cardiovascular outcomes. Although placebo-control clinical studies are important in generating evidence for the efficacy of an intervention, real-world data are equally important because they eliminate the inherent selection bias of clinical trials and reflect real prescribing practice.
The present study enrolled diabetic patients started with one or more diabetic medication and many factors including medication adherence, lifestyle, dietary habits and over the counter medication may affect HbA1c reduction level, as well as eGFR levels. However, the results may provide a preliminary research basis for a larger scale study with a longer followed-up period. Furthermore, the relatively small sample size and short follow-up duration limited the study to predict long term cardiorenal protective effects of empagliflozin. A larger-scale, multicenter clinical study recruiting diabetic patients with high HbA1c and coronary heart disease or with chronic kidney disease is needed to confirm this conclusion.
IBM SPSS Statistics, version 26 (RRID: SCR_019096), is available to the researchers in the study and they have access to the software under the license provided to UAE university.
This article presents subgroups analysis of the original trial that was registered at the US National Institutes of Health (ClinicalTrials.gov protocol registration: NCT04942119) and the original trial was approved by the Abu Dhabi Health Services Company (SEHA) Research Oversight and Ethics Committee (SEHA REC) in the United Arab Emirates (approval number: SEHA-IRB-021). Informed consent was obtained from all participants before study recruitment and they received a copy of it along with the study information sheet.
All authors had access to research data and contributed to the writing and review of the manuscript.
All authors have disclosed that they have no financial interests or any significant relationship with or in any other institutes related to this research or article.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
We would like to acknowledge Oud Al Touba diagnostic and screening centre staff for their support during the study.