AE Sri Ajjayya1, T Aman Suresh2*, Omkarswamy Maradimath1
Department of Pharmaceutics and SJM College of Pharmacy, Chitradurga, Karnataka, India
Department of Pharmacy Practice- Sandip institute of Pharmaceutical Sciences, Nashik, Maharashtra, India
Received: 02 March 2024; Processed: 25 May 2024; Accepted: 03 June 2024
Citation: Suresh, Aman T., et al. “Microsponges: An Advanced Drug Delivery System.” J Pharmacol Drug Deliv (2024): 104. DOI: 10.59462/JPDD.2.1.103
Copyright: © 2024 Suresh AT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
In the field of drug delivery system, there are so many new formulation techniques. Microsponges are one of the recent unique techniques which are gaining popularity now days due to their use of controlled release and targeted drug delivery system. Microsponges hold a certification as one of the potential approaches for gastric retention where many oral dosage forms face several physiological restrictions due to non-uniform absorption pattern, inadequate medication release and shorter residence time of the dosage form in the stomach. This article presents broad review of microsponges delivery system discussing the principle and preparation method. Appropriate analytical technique for characterization of microsponges like particle size and its distribution, morphology, porosity, surface, density are covered.
Microsponges• Drug delivery• Oral administration• Transdermal delivery
The major challenge to the pharmaceutical industry is to control the delivery rate of active pharmaceutical ingredient to a pre-determined site in human body. Here researcher focused on designing different controlled release drug delivery systems to improve safety, efficacy, and patient compliance [1]. Several predictable and reliable systems were developed for systemic drugs in the heading of transdermal delivery system (TDS) using the skin as portal of entry. It has improved the efficacy and safety of many drugs that may be better administered through skin. But TDS is not practical for delivery of materials whose final target is skin itself [2].
Microsponges are polymeric delivery system composed of porous microspheres which having a particle size range of 5-300 μm with a capability to entrap a wide range of active ingredients and are used as a carrier for topical drug delivery as shown in (Figure 1) (Junqueira and Bruschi 2018). Microsponges are tiny sponge-like spherical particles that consist of a myriad of interconnecting.
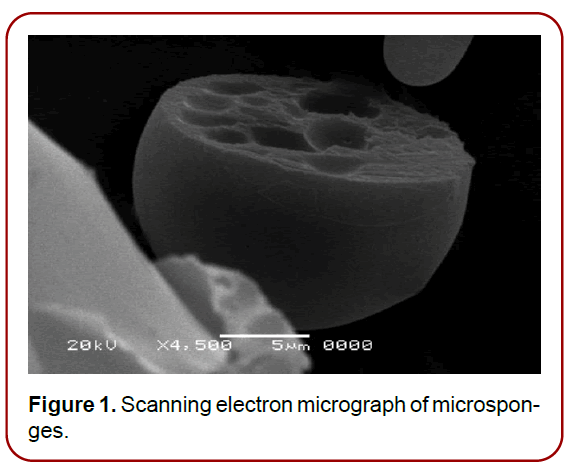
Figure 1. Scanning electron micrograph of microsponges.
Applications of microsponge systems
Topical delivery: Topical agents are a mainstay in cosmetics and the treatment of dermatological disorders. However, they are associated with substantial skin irritancy, especially in sensitive patients. The rapid release and subsequent accumulation of the active ingredients of the topical agents have been associated with this irritancy. Microsponge delivery technology provides controlled release of the active ingredients onto the skin. Several microsphere-based topical agents have been evaluated for their safety and efficacy for cosmetic purposes and in the treatment of dermatological disorders, and are currently marketed in the US. These include formulations of benzoyl peroxide, tretinoin acid, HQ plus retinol, and 5-FU. Formulations of topical agents utilizing the Microsponge drug delivery system technology have shown little or no irritancy in patients with acne, photo damaged skin, hyper pigmentation, without sacrificing the efficacy of the agents [4].
Oral delivery: In oral drug delivery the microsponge system increase the rate of solubilization of poorly watersoluble drugs by entrapping them in the microsponge system’s pores. As these pores are very small the drug is in effect reduced to microscopic particles and the significant increase in the surface area thus greatly increase the rate of solubilization. A Microsponge system offers the potential for active ingredients to remain within a protected environment and provide controlled delivery of oral medication to the lower gastrointestinal (GI) tract, where it will be released upon exposure to specific enzymes in the colon. If this approach is successful then it should open up entirely new opportunities for Microsponge drug delivery system. It has been shown that microsponge system enhances the solubilization of drugs which are poorly soluble by entrapping these drugs in their pores [5].
Bone substitutes: Bone-substitute compounds were obtained by mixing pre-polymerized powders of polymethylmethacrylate and liquid methyl methacrylate monomer with two aqueous dispersions of a-tri calcium phosphate (a-TCP) grains and calcium-deficient hydroxyapatite (CDHA) powders. The final composites appeared to be porous. Osteo-conductivity and Osteoconductivity of the final composites were tested In-vivo by implantation in rabbits. Formation of new trabecular bone was observed inside the pores where the inorganic powders had been placed. The material produced shows a good level of biocompatibility, good osteointegration rate and osteogenetic properties [6].
Cardiovascular engineering using microsponge technology: Biodegradable materials with autologous cell seeding, requires a complicated and invasive procedure that carries the risk of infection. A biodegradable graft material containing collagen microsponge that would permit the regeneration of autologous vessel tissue has developed. The ability of this material to accelerate in-situ cellularization with autologous endothelial and smooth muscle cells was tested with and without pre-cellularization. Poly (lactic-co-glycolic acid) as a biodegradable scaffold was compounded with collagen microsponge to form a vascular patch material. Histological results showed the formation of an endothelial cell monolayer, a parallel alignment of smooth muscle cells, and reconstructed vessel wall with elastin and collagen fibres. The cellular and extracellular components in the patch had increased to levels similar to those in native tissue at 6 months. This patch shows promise as a bioengineered material for promoting in situ cellularization and the regeneration of autologous tissue in cardiovascular surgery [7].
Microsponges for biopharmaceuticals delivery: The microsponge delivery system is employed for both in the delivery of biopharmaceuticals as well as in tissue engineering. Dai 2010 et al developed 3D scaffolds hybrid structures that have advantages of natural type I collagen and synthetic PLGA knitted mesh. The collagen microsponges facilitated cell seeding and tissue formation and mechanically strong PLGA mesh served as a skeleton. The scaffolds were divided into three groups: a) Thin: collagen microsponge formed in interstices of PLGA mesh; b) Semi: collagen microsponge formed on one side of PLGA mesh; c) Sandwich: collagen sponge formed on both sides of PLGA mesh [8].
The drug release profile of microsponges delivery system
These are designed in a way to drug release within the definite period as a result of external stimulus. [9, 10]. (Figure 2) shows various triggered-based mechanisms employed for drug release in MSD.
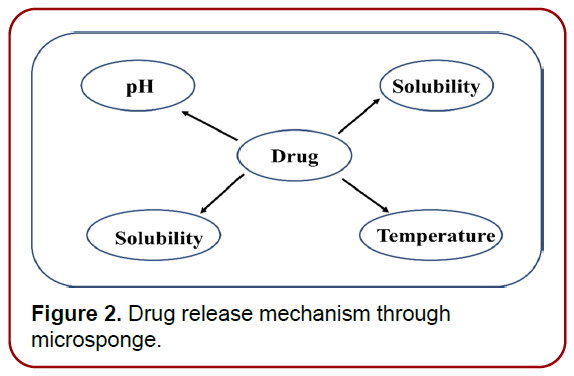
Figure 2. Drug release mechanism through microsponge.
pH‑stimulated system: By altering the coating on the MDS, pH-dependent drug release is enabled.
Change of temperature: At room temperature, the entrapped drug may be too viscous to move rapidly from the microsponge to the dermis. However, when the temperature of the skin increases, the rate of active flow increases, and hence the rate of release increases as well.
Pressure: Applying pressure while rubbing can result in the release of drugs encapsulated in MDS onto the skin. The amount of gas released is governed by the sponge’s quality Microsponge formulations can be improved by altering the material used and a number of other variables.
Solubility: Water-miscible chemicals (antiperspirants and antiseptics) will release additives in the presence of water. Additionally, the diffusion mechanism may initiate the drug’s release [11].
Engineering of MDS: Apart from the microsponge composition, the delivery system’s performance is influenced by the preparation processes used. However, due to their complexity and expense, microsponge preparation methods are restricted. Drug release can be performed in two ways: one-step process and two-step process (liquid– liquid polymerization) and quasi-emulsion diffusion method. Both methods are dependent on the physicochemical properties of the active drug that must be entrapped; for example, if the drug is a non-polar and inert material, it will produce a structure that is porosity, as shown in [Figure 3] Porogen drugs that do not inhibit polymerization and do not trigger it, as well as being stable towards free radicals, may be entrapped using a one-step procedure [10, 12].
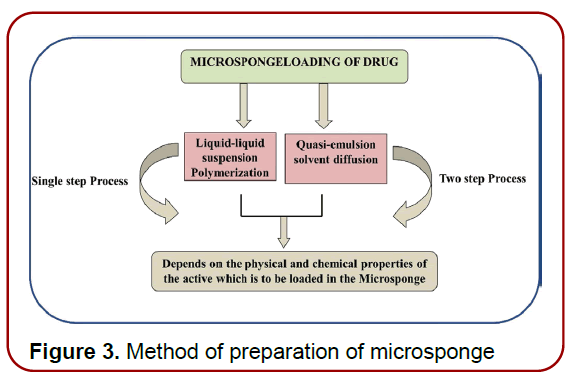
Figure 3. Method of preparation of microsponge
Method of Preparation
Liquid–liquid suspension polymerization: Typically, a solution containing monomers and active additives that are water-immiscible is created first. (Figure 4) summarises the number of stages involved in liquid– liquid suspension polymerization processes [13]. This water-soluble phase is suspended in water with agitation and often comprises chemicals such as emulsifiers and dispersants. Polymerization is achieved byactivating monomers with the assistance of catalysis, temperature elevation, or irradiation after the solution has been formed with separate droplets of the desired size. The polymerization technique results in the formation of 1000 cages of MDS with spherical structures that are linked and resemble a bunch of grapes. After the polymerization is finished, the solid particles will be collected from the resulting suspension. After that, the particles were rinsed and dried before being used again [12,14].
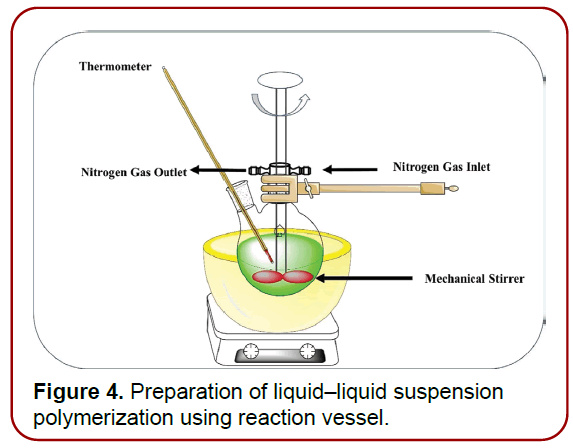
Figure 4. Preparation of liquid–liquid suspension polymerization using reaction vessel.
Quasi‑emulsion solvent diffusion: The procedure described here is commonly used to make topical and oral microsponges. The procedure involves the production of two phases, one of which is the inner organic phase, which contains the drug, and the other is the outer aqueous phase, which is then agitated and filteredbefore being used. The inner phase is then blended drop by drop in the outer phase with the assistance of a mechanical stirrer for 60 min. The quasi-emulsion droplets were formed as a result of continual stirring, and the solid cages of microsponge were formed as a result of further organic solvent evaporation. After that, the microsponges were filtered and dried in the oven for12 h. (Figure 5) summarizes the steps required in microsponge production utilising the approach quasi-emulsion solvent diffusion [15–16].
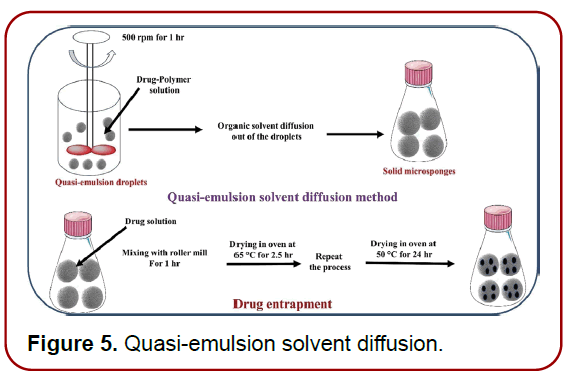
Figure 5. Quasi-emulsion solvent diffusion.
Multiple‑emulsion solvent diffusion method: The approach was developed to create porous and biodegradable microspheres. An aqueous inner phase was used with the addition of stearyl amine, and the span was distributed in solution. This w/o emulsion is then dispersed again in an aqueous phase with polyvinyl alcohol to generate (w/o/w) double emulsion. This method reveals the advantage of capturing both soluble and insoluble actives. This method may also be used to entrap thermolabile compounds like proteins [12].
Evaluation of microsponge: Evaluation of microspnge is done by dissolution drug-release studies using USP basket apparatus at 37 °C ± 0.5 °C [17]. The USP Basket has a stainless-steel mesh of 5 μm pore size. The rotation speed of basket is 150 rpm. The surface characteristics and the morphology of the microsponge are evaluated by scanning electron microscopy. Particle size of microsponges can be determined by laser light diffractometry. True density determination is done by an ultra-pycnometer under helium gas [18-21]. Microsponge pore diameter can be calculated using the Washburn equation [22].
Compatibility studies of microsponge are done using thin-layer chromatography and infra-red spectroscopy. Viscoelastic properties need to be defined according to the microsponge requirements for producing caplet that is flexible or crippled. In vitro diffusion studies of microsponge are done using a Keshary–Chien diffusion cell (cellophane membrane). In this, a receptor compartment is used i.e., 100 mL of phosphate buffer and 500 mg gel loaded 10 mg of a drug are dispersed on the cell membrane. Then, this is maintained at 37 °C ± 0.5 °C temperature for a predetermined time interval stirring the solution using Teflon-coated magnetic bars at 5 mL of solution is now removed from the compartment and 5 mL of fresh phosphate buffer is added. Finally, the drug concentration is measured by using spectrophotometrically with comparing blank solution. This process is repeated three times [23-25].
Characteristics of microsponges
• Formulations are stable over range of pH 1 to 11.
• Microsponge formulations are stable at the temperature up to 130oC.
• Microsponge formulations are compatible with most vehicles and ingredients.
• Microsponge formulations are self-sterilizing as their average pore size is 0.25μm where bacteria cannot penetrate.
• Microsponge formulations have higher payload (50- 60%), still free flowing and can be cost effective [26].
Advantages
• Advanced oil control, absorb up to 6 times its weight without drying.
• Extended release.
• Reduced irritation formulas.
• Allows novel product form.
• Improved product aesthetics.
• Extended release, continuous action up to 12 h.
• Reduced irritation, better tolerance means broader consumer acceptance.
• Improved product aesthetics, gives product an elegant feel.
• Improves stability, thermal, physical and chemical stability.
• Allows incorporation of immiscible products.
• Improves material processing eg. liquid can be converted to powders.
• Allows for novel product forms.
• Improves efficacy in treatment.
• Cure or control confirm more promptly.
• Improve control of condition.
• Improve bioavailability of same drugs [27].
Microsponge delivery system can be a winning strategy for a new generation of Pharmaceutical and Cosmetic industry. Microsponges have a distinct advantage over the existing conventional topical dosage forms for the treatment of tropical diseases; it is a unique technology for the controlled release of topical agents also use for oral as well as biopharmaceutical drug delivery. These systems also provide tremendous opportunities in the designing of new controlled and delayed release oral formulations, thus extending the frontier of futuristic pharmaceutical development. Increased sophistication of this system will ensure the successful advancements in the avenue of gastro retentive microsponge therapy to optimize the delivery of molecules in a more efficient manner. The Microsponge drug delivery system offers entrapment of its ingredients and is believed to contribute toward reduced side effects, improved stability, increased elegance, and enhanced formulation flexibility. In addition, numerous studies have confirmed that microsponge systems are nonirritating, non-mutagenic, non-allergenic, and nontoxic. This kind of drug delivery technology may lead to a better understanding of the healing of several diseases.