Zhengrui Li1, 2, 3, 4, 5, 6, 7, 8, 10,*, Jing Li9,10, Xufeng Huang9,*
Department of Oral and Maxillofacial-Head and Neck Oncology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China;
College of Stomatology, Shanghai Jiao Tong University, Shanghai, China;
National Center for Stomatology, Shanghai, China;
National Clinical Research Center for Oral Diseases, Shanghai, China;
Shanghai Key Laboratory of Stomatology, Shanghai, China;
Shanghai Research Institute of Stomatology, Shanghai, China.
Shanghai Center of Head and Neck Oncology Clinical and Translational Science, Shanghai, China.
Research Unit of Oral and Maxillofacial Regenerative Medicine, Chinese Academy of Medical Sciences, Shanghai, China.
Ministry of Education-Shanghai Key Laboratory of Children’s Environmental Health, School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Faculty of Dentistry, University of Debrecen, 4032 Debrecen, Hungary.
Received: 15 November 2023; Accepted: 30 November 2023; Published: 28 December 2023
Citation: Li Zhengrui, Li Jing and Huang Xufeng, “Sarcopenia and its Impact on the Immune System.” Immunol Res Immunother (2023): 101. DOI: 10.59462/IRIT.1.1.101.
Copyright: © 2023 Li Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The phenomenon of sarcopenia, characterized by a progressive decline in skeletal muscle mass and function, epitomizes a significant aspect of the aging process. Current estimates suggest that sarcopenia affects approximately 50 million individuals globally, a figure projected to escalate to 500 million by 2050. This condition, often insidious in its onset, precipitates substantial functional impairments, elevating the risk of falls, disability, and mortality among the elderly. Consequently, sarcopenia substantially diminishes the quality of life for older adults and imposes a considerable burden on healthcare systems worldwide (Consensus of Diagnosis and Treatment of Sarcopenia in Chinese Older Adults, 2021) (Figure 1).
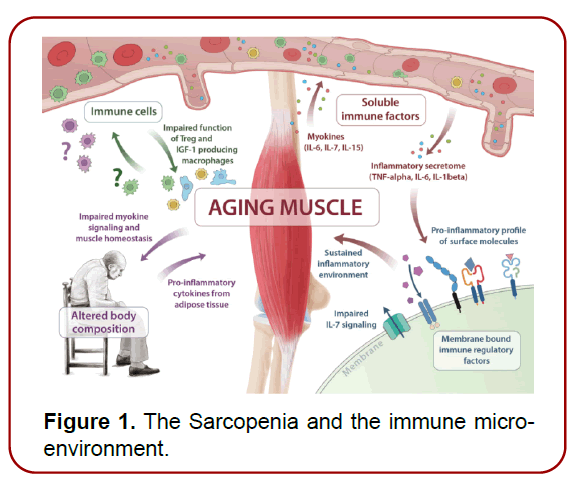
Figure 1. The Sarcopenia and the immune microenvironment.
The Interplay between Sarcopenia and Immunosenescence
With advancing age, the human immune system undergoes a process of senescence known as immunosenescence, often accompanied by chronic low-grade inflammation, which can contribute to muscle atrophy. This muscleimmune system relationship is inherently bidirectional. Chronic inflammation prompts a muscle catabolic metabolism, driven by the secretion of inflammatory cytokines. Conversely, a significant portion of skeletal muscle maintains immune health. However, dysregulation in this system, manifested through altered myokine signaling, pro-inflammatory membrane-bound factors, and compromised regenerative capacity of immune cells, can lead to immune dysfunction.
Biological aging disturbs the delicate balance between muscle and immune system homeostasis. Inactivity, metabolic changes, and chronic inflammation progressively impair muscle functionality, which in turn disrupts immune regulation and skeletal muscle signaling. This establishes a detrimental cycle, particularly evident in sarcopenia patients, where compromised immune function heightens susceptibility to infections, including COVID-19. (Figure 2).
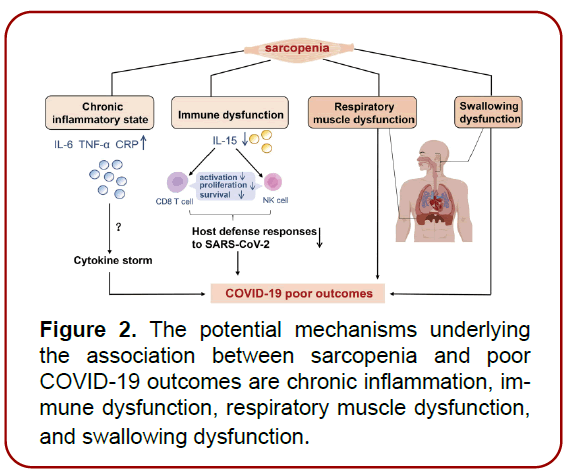
Figure 2. The potential mechanisms underlying the association between sarcopenia and poor COVID-19 outcomes are chronic inflammation, immune dysfunction, respiratory muscle dysfunction, and swallowing dysfunction.
The ramifications of sarcopenia on autoimmune diseases and cancer immune response remain to be fully elucidated. Although there is a concurrent increase in these diseases and a decrease in muscle mass with age, causality remains speculative. Notably, during aging, serum levels of proinflammatory cytokines like CRP, TNF-α, and IL-6 increase, with IL-6 being a pivotal predictor of sarcopenia. Extended exposure to IL-6 and associated cytokines exacerbates the pro-inflammatory effects and muscle catabolism inherent to the IL-6 signaling pathway. Furthermore, sarcopenia impairs the anti-inflammatory responses typically induced by IL-6 post-exercise, hampering muscle synthesis metabolism. Elevated serum levels of TNF-α and CRP are also correlated with sarcopenia.
Additional Factors Influencing Muscle Aging
Anabolic Resistance: In elderly muscles, there is a noted prevalence of anabolic resistance (AR), a condition characterized by a diminished response to muscle growth stimuli such as muscle contraction, dietary amino acids, and anabolic hormones like IGF-1. This resistance results in lower protein synthesis rates and a reduced capacity for anabolic metabolic response, necessitating higher dietary protein intake for older individuals. The reduced sensitivity of the mTOR-AKT pathway is a principal molecular mechanism underlying AR. (Figure 3)
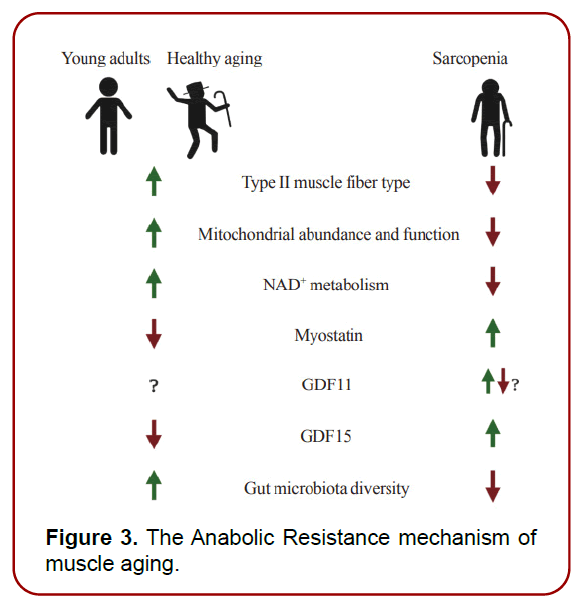
Figure 3. The Anabolic Resistance mechanism of muscle aging.
Muscle Fiber-Type Alterations: Aging induces shifts in muscle fiber types, with a decline in the number and size of type II fibers and a consistent level of type I fibers. Consequently, aging skeletal muscles transition from glycolytic to oxidative metabolism, increasing the demand for functional mitochondria.
Mitochondrial Dysfunction: This refers to the decline in mitochondrial functionality due to structural damage, impaired metabolism, mitochondrial DNA damage, and imbalances in mitochondrial dynamics. Mitochondrial dysfunction is a recognized hallmark of aging and is intimately linked with sarcopenia in skeletal muscles and motor neurons.
Decline in NAD+ Metabolism: NAD+ plays a crucial role in various metabolic processes. Aging and pathological conditions lead to reduced NAD+ levels in tissues, including skeletal muscles. Supplementing NAD+ precursors has shown promise in enhancing muscle cell regenerative capacity and mitigating muscle atrophy.
In conclusion, sarcopenia, as a critical aspect of the aging process, presents a multifaceted challenge that extends beyond mere muscle loss. Its intricate interplay with immunosenescence signifies a broader impact on the immune system, highlighting the bidirectional relationship between muscle degradation and immune functionality. The escalation of pro-inflammatory cytokines and the emergence of anabolic resistance with advancing age further exacerbate this condition. This complex interaction underscores the necessity for a holistic approach in addressing the health of the elderly population. Understanding the mechanisms underpinning sarcopenia, including the pivotal role of mitochondrial dysfunction and changes in muscle fiber composition, is essential for developing effective therapeutic strategies. The potential of NAD+ metabolism in mitigating the effects of sarcopenia opens new avenues for research and intervention. Ultimately, addressing sarcopenia is not only about managing muscle loss but also about enhancing the overall health and quality of life in our aging population.