Ramsha Haider1*, Amna Mujtaba1, Fatema Ali Asghar1, Kinza Tanveer1, Rumaisa Khokhar1 and Wajiha Ahmed2
MBBS, Karachi Medical and Dental College, Karachi, Pakistan
MBBS, Sindh Medical College, Jinnah Sindh Medical University, Karachi, Pakistan
Received: 13 June 2024; Accepted: 01 July 2024; Published: 07 July 2024
Citation: Haider Ramsha. “The Efficacy of Laser Interstitial Thermal Therapy (LITT) in Drug-Resistant Temporal Lobe Epilepsy: A Review.” J Fam Med Clin Res (2024): 103. DOI: 10.59462/JFMCR.1.1.103
Copyright: © 2024 Haider R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Importance: Temporal Lobe Epilepsy (TLE) is a prevalent condition impacting numerous individuals globally and drug-resistant cases present a formidable obstacle. Conventional surgical interventions come with inherent risks and potential cognitive repercussions due to the necessity of resecting brain tissue. Laser Interstitial Thermal Therapy (LITT) has surfaced as an innovative, minimally invasive substitute that prioritizes the preservation of vital cerebral areas while striving to achieve seizure control.
Observation: Epilepsy, a condition affecting more than 68 million people worldwide, often remains unresponsive to medication, leading to the exploration of surgical options. Among these, mesial temporal lobe epilepsy (mTLE) is prevalent and conventional procedures like Anterior Temporal Lobectomy (ATL) and selective amygdalohippocampectomy have their drawbacks. Laser Interstitial Thermal Therapy (LITT), guided by magnetic resonance imaging (MRI), has emerged as a less invasive alternative, minimizing infection risks, hospitalization duration, and complications. It has also proven effective in treating both tumors and drug-resistant mTLE. LITT provides comparable seizure control to traditional surgeries while offering benefits such as reduced cognitive impact, shorter hospital stays, and improved quality of life.
Conclusion and Relevance: In a world where millions suffer from epilepsy, the emergence of MRI-guided LITT provides hope. This research aligns with the changing epilepsy treatment landscape, emphasizing the importance of exploring innovative and minimally invasive options. LITT has the potential to advance medical science and elevate patient care. LITT marks a revolutionary shift in epilepsy treatment diverging from traditional open surgery. LITT’s minimally invasive approach has yielded impressive results. LITT represents a beacon of hope for individuals grappling with drug-resistant epilepsy, promising enhanced patient outcomes and a significantly improved quality of life.
Temporal Lobe Epilepsy (TLE) is one of the most common forms of drug-resistant epilepsy, affecting a significant number of patients worldwide. Traditional surgical interventions, such as anterior temporal lobectomy or selective amygdalohippocampectomy, have shown promising results in controlling seizures. However, these procedures may involve significant risks and cognitive side effects due to the resection of critical brain tissue.
Laser Interstitial Thermal Therapy (LITT) has emerged as an innovative and minimally invasive alternative to traditional surgical approaches in the treatment of drugresistant TLE. LITT involves the application of laser energy to ablate the epileptogenic brain tissue, thereby aiming to achieve seizure control while preserving important brain regions.
Epilepsy is a global concern affecting more than 68 million people worldwide [1,2]. It commonly manifests in childhood and old age, with approximately half of all cases emerging in these age groups. In 30% of cases, seizures are deemed to be resistant to medical management as clinically available anticonvulsant drugs are inefficient in controlling seizures in around 30% of epileptic patients necessitating alternative approaches like surgical intervention [3].
Temporal lobe epilepsy (TLE); due to its prevalence and well-known drug resistant properties is one of the most common forms of medically refractory epilepsy referred for surgical management. Within TLE cases, mesial temporal lobe epilepsy (mTLE) accounts for the majority, around 70% [4,5]. While surgical alternatives include Anterior temporal lobectomy (ATL) for mesial temporal lobe epilepsy and Selective Amygdalohippocampectomy has been the standard treatment for patients with TLE or mLTE while effective for some, come with notable drawbacks [6-8], the surgical invasiveness of traditional surgical approaches, including cognitive dysfunction, visual field defects, and intracranial bleeding, may lead to patient deterrence [4]. In light of these challenges, there has been a recent shift toward exploring less invasive and cortex-preserving techniques. Among these, laser interstitial thermal therapy (LITT) gained traction. This method employs low-voltage laser energy through optical fibers to thermally ablate the source of epileptic activity, all under real-time guidance from magnetic resonance imaging (MRI), known as MRguided LITT (MRgLITT). Furthermore, LITT allows for a reduced risk of infections and leads to shorter hospital stays and decreased overall complications. Notably, LITT showcases efficacy in treating not only epilepsy but also tumors in both adult and pediatric patients, with shorter hospitalizations and lower morbidity. Advantages of LITT over other minimally invasive procedures such as stereotactic radiosurgery include real-time visualisation of the ablation with MRI thermometry, discrete lesion margins, and lower infection rates [9].
The primary objective of this narrative review is to assess the efficacy and safety of Laser interstitialThermal Therapy (LITT) as a surgical treatment option for drugresistant temporal lobe epilepsy. By comparing LITT with traditional surgical approaches, this review aims to provide a comprehensive analysis of the outcomes in terms of seizure control, post-operative complications, cognitive effects, and overall quality of life for patients with drugresistant TLE.
In this paper we evaluate multiple literary sources to determine the efficacy of LITT in drug resistant TLE. LITT’s minimally invasive nature, guided by MRI, and its potential to effectively manage seizures while minimizing complications have garnered attention as a promising solution for drug-resistant epilepsy cases, particularly those attributed to mesial temporal lobe epilepsy.
We performed a comprehensive literature search across PubMed, Google Scholar, and Sci-Hub databases, yielding a total of 52 relevant articles. These articles served as the foundation for developing the manuscript of our review study. We used information from the articles and have referenced them throughout the paper, to evaluate the Efficacy of Laser Interstitial Thermal Therapy in Drug- Resistant Temporal Lobe Epilepsy.
LITT procedure and parameters
LITT is a technique that employs laser delivery through optical fibers to irreversibly ablate target tissue using heat. Long optical fibers connect the patient to an external laser source. During LITT, a diffusing tip, typically around 1 cm long, introduces laser light into the patient’s tissue. Innovative imaging methods such as magnetic resonance (MR) thermography can be combined with LITT to visualize the target tissue. This enables surgeons to plan laser trajectories effectively, optimize laser positioning, and provide real-time assessment of thermal damage during the procedure [10].
In the context of LITT, laser-induced thermal therapy selectively targets and destroys tumor cells by delivering precise heat. This is achieved by using an Nd:YAG laser of 1064 nm wavelength through optical fibers. This laser can penetrate tissue to depths of 2 to 10 mm. When aimed at the tumor, it transforms laser photons into thermal energy within the tumor tissue [10].
For effective results, maintaining tissue temperature between 43 °C and 45 °C for at least 10 minutes makes cancer cells more responsive to subsequent chemotherapy and radiation therapy. Alternatively, temperatures ranging from 50 °C to 80 °C for a shorter duration cause tumor necrosis through protein denaturation. Importantly, the extent of tissue damage is contingent on both the temperature and the duration of thermal energy application. This assessment is aided by the Arrhenius thermal dose model, which estimates tissue damage based on a specific temperature and time range (25 to 240 minutes at 43 °C). Furthermore, this model is made more effective through the use of modern MRI software, which generates thermal maps to visually track these changes, thus facilitating the monitoring of tumor necrosis. To ensure complete tumor destruction, it’s crucial to monitor temperature during ablation. However, the presence of cerebrospinal fluid spaces or nearby blood vessels can dissipate heat from the ablation area, creating a heat sink effect. Consequently, this effect can either lead to incomplete ablation or, conversely, provide insulation to safeguard nearby vital structures from thermal damage. Moreover, monitoring thermal changes in lesions containing fat presents particular challenges due to the influence of fat’s chemical composition on the accuracy of MRI temperature readings [11].
Data from retrospective studies imply that it may benefit patients with drug-resistant epilepsy stemming from conditions like mesial temporal sclerosis, hypothalamic hamartoma, cavernous hemangioma, and small cortical dysplasias and malformations. In some centers, it’s even being considered as an initial treatment instead of anterior temporal lobectomy, with ongoing prospective studies comparing its outcomes with traditional temporal lobectomy [12].
The procedural sequence for conducting Laser Interstitial Thermal Therapy (LITT) surgery to address epilepsy involves several distinct stages. Firstly, anesthesia and patient positioning are determined based on patient preference and procedural requirements, with options ranging from light sedation to general anesthesia. Subsequently, preoperative imaging, such as a CT scan or MRI, is performed to ascertain the size, location, and characteristics of the epileptic focus. These detailed images guide the medical team in planning the optimal path and positioning for the laser fiber during the procedure, ensuring real-time accuracy while safeguarding surrounding healthy tissue [13].
Next, using intraoperative neuronavigation, a small burr-hole is meticulously created with a twist drill, and a bone anchor is precisely positioned in the skull along the neuronavigated target trajectory. The cooling catheter is then inserted through the bone anchor, reaching the intended target site and securely affixed to the anchor. Following this, the laser probe is introduced into the cooling catheter and securely locked in place. T2 imaging is performed to confirm the precise positioning of the laser probe, ensuring utmost accuracy, while baseline images using fast-spoiled gradient recalled phase are acquired at the patient’s body temperature [14].
The procedure advances with the initiation of the cooling system’s circulation, followed by a test pulse of 3–4 W for 30–60 seconds. This step aids in pinpointing the location of the distal 1-cm segment of the laser probe, which is pivotal for accurate ablation. Subsequently, the ablation treatment is administered by applying doses of 10–15 W for 30–180 seconds, aiming to encompass the entirety of the target lesion within the damage zone [14].
Once the ablation is completed, the laser probe, cooling catheter, and anchor are meticulously removed. The small skin puncture site is then closed using a continuous Monocryl stitch, concluding the procedure. The duration of hospitalization following the procedure may vary, but typically ranges between 24 to 48 hours for uncomplicated cases, allowing for adequate postoperative monitoring and recovery [14], (Figure 1).
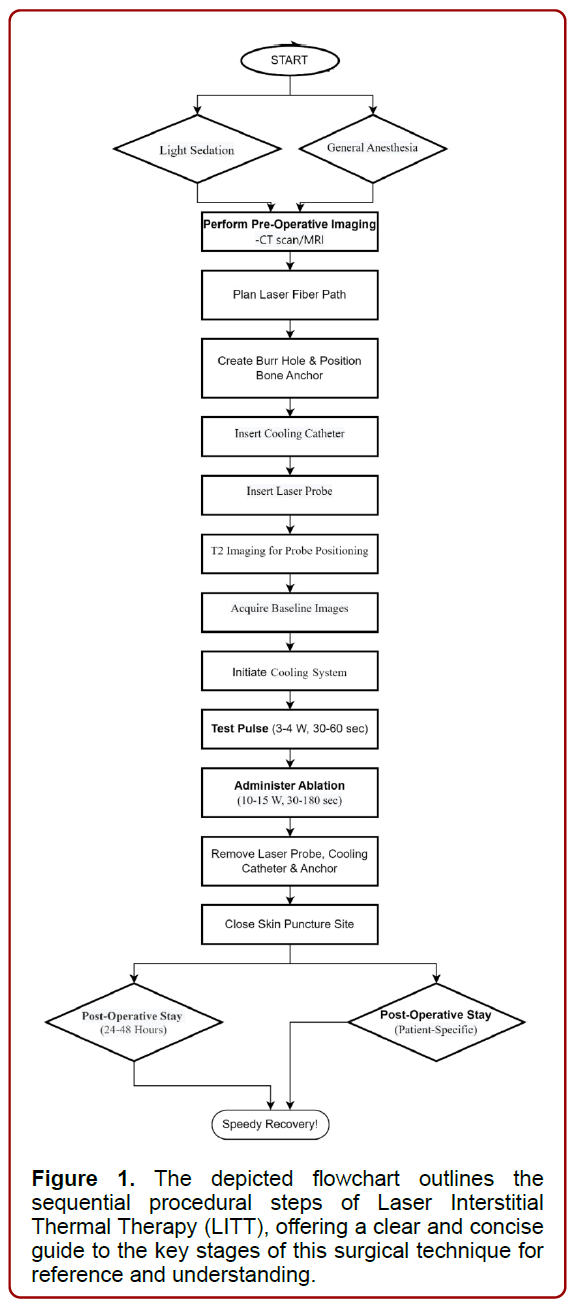
Figure 1. The depicted flowchart outlines the sequential procedural steps of Laser Interstitial Thermal Therapy (LITT), offering a clear and concise guide to the key stages of this surgical technique for reference and understanding.
Comparative studies
In a comparative analysis of epilepsy surgical techniques, one Randomized Controlled Trial (RCT) found that Selective Amygdalohippocampectomy (SAH) resulted in significantly lower seizure-free outcomes at 12 months compared to other techniques [15]. However, four other studies did not report significant differences between these methods [16]. Notably, a pediatric study showed that Anterior Temporal Lobectomy (ATL) had significantly better seizure outcomes after an average follow-up of 5.3 years [17]. An analysis conducted included studies comparing ATL with Radiosurgery and Radiofrequency [18]. The study involved 2,183 patients, with 1,248 undergoing ATL and 935 undergoing SAH (including transcortical, transsylvian, and unspecified approaches) [18].
LITT presents a promising option for epilepsy treatment, with seizure outcomes comparable to open surgery. Visual field defects are common complications. Radiofrequency therapy has a lower seizure-free rate and limited evidence. Radiosurgery, while outpatient-friendly, is less effective than open surgery. It’s essential to conduct formal visual testing after epilepsy surgery, and LITT’s shorter hospital stay makes it an attractive alternative [15,19,20,21].
However, more research and long-term comparisons are needed for firm conclusions about LITT’s effectiveness. Table 1
| NAME OF STUDY | YEAR | STUDY TYPE / DESIGN |
METHOD | KEY FINDINGS |
|---|---|---|---|---|
| Schramm J et al (6) |
2011 | Randomised control trial | The study aimed to create two major patient groups, SAH and TLR, and within each group, there were further subgroups randomised for mesial resection lengths. One subgroup was designated for a minimal resection of 25 mm, while the other subgroup had a planned resection of at least 35 mm. This approach resulted in four distinct subgroups, allowing for a comparison of different resection lengths within the SAH and TLR patient categories. |
The study findings suggest that SAH may not be the optimal surgical technique for achieving seizure-free outcomes at the 12-month mark, as it demonstrated significantly lower success rates when compared to other surgical approaches. |
| Lee T et al (7) | 1997 | Clinical trial | 38 patients who underwent left temporal lobe surgery for epilepsy were involved. Epileptogenic focus localization was determined using a combination of video and scalp EEG recordings, neuropsychological evaluations, MRI, intracerebral or subdural EEG recordings. Among the participants, 25 underwent conventional ATL, and 13 received selective AH. |
The outcomes favoured the ATL group in becoming seizure free compared to SAH. Verbal memory was compromised post operatively in both groups while visual performances and verbal fluency showed improved signs in postoperative follow ups. |
| Elliot CA et al (8) |
2018 | Clinical Trial | A retrospective analysis of assessment of postoperative seizure outcomes in cases involving medically resistant paediatricTLE where the initial treatment options were either SAH or ATL. | The paediatric study's results highlight the effectiveness of ATL as a treatment option for epilepsy in children, with significantly improved seizure outcomes observed after an average follow- up of 5.3 years. This underscores the potential long-term benefits of ATL in managing seizures in paediatric patients. |
| Barbaro NM et al (9) | 2018 | Randomised controlled ROSE trial | The study assessed efficacy of SRS stereotactic radiosurgery versus standard ATL treatment for mLTE in adults in post-operative management of seizure remission, verbal memory, and quality of life at the 36-month follow-up. | Study indicates that ATL outperforms the minimally invasive option of SRS in terms of both the speed of effectiveness and the occurrence of adverse events, as well as the percentage of patients experiencing seizure remission. |
| A l o n s o Vanegas et al (10) | 2018 | Comparative clinical trial | Adult patients with medically refractory mTLE-HS epilepsy who underwent an ATL, trans t-3 SAH, or PHC from 2008-2011 were assessed based on different postoperative outcomes |
The preliminary investigation observed that PHC, ATL, and SAH demonstrated comparable effectiveness in achieving short- term seizure-free outcomes among patients with mTLE- HS. While , PHC did not result in postoperative visual field deficits, over the long term, its efficacy appeared to decrease in comparison to the other surgical approaches |
| Foged MT et al (12) | 2018 | Clinical trial | A total of 56 patients were examined in the study; divided into two groups: 22 underwent SAH, and 34 underwent TLR for MLTE due toHS. The evaluation of seizure outcomes followed the Engel classification at both 1 year and 7 years post- surgery. Patients categorised in Engel class I, indicating seizure freedom, were compared with those in Engel classes II to IV. |
The study reveals that among individuals suffering from drug-resistant focal MTLE characterised by HS SAH leads to enduring freedom from seizures and improved memory function in comparison to patients who undergo non-selective TLR. This implies that SAH offers a more favourable outcome for patients facing this specific form of epilepsy and HS. |
| Marathe K et al (13) | 2021 | Systematic review and Meta-analysis | 41 studies from different databases were included based on MTLE that reported seizure-free results for at least 10 patients with a follow-up period of at least 12 months. The surgical approaches considered included trans-sylvian and transcortical SAH, TLR, LITT, TSA and RF-TC to compare the effectiveness and impact of these interventions and to estimate the likelihood of seizure freedom per person-year. |
Results indicated that The seizure-free rates per person- year were most promising in trans-sylvian SAH, TLR and transcortical SAH. While minimally invasive approaches such as LITT, TLR, and RF-TC generally exhibited lower efficacy compared to open surgery due to shorter follow-up periods with RF-TC proving to be the least impactful. |
Table 1. This table evaluates and summarizes various studies regarding LITT; in a tabular form.
Seizure control efficacy
Laser Interstitial Thermal Therapy (LITT) presents a less risky, minimally invasive option compared to conventional surgery for treating drug-resistant epilepsy, particularly when it comes to cognitive concerns. Earlier research investigated the usefulness of LITT for controlling MTLE. About 55% of cases achieved freedom from seizures, and this result wasn’t influenced by patient age [22].
Anterior temporal lobectomy and amygdalohippocampectomy (ATL) are considered the effective surgical interventions for treating drug-resistant mesial temporal lobe epilepsy (mTLE). However, it’s not uncommon for seizures to return even after undergoing ATL surgery. Previous studies show a patient with recurring seizures after ATL found long-term relief through successful LITT, targeting the remaining hippocampus. If proven safe and effective, this could be valuable for certain post-ATL mTLE patients. LITT showed nearly double the seizure-free duration compared to ATL. Since the patient was seizure-free for nearly 2 years post-ATL, longer followup is needed to assess LITT’s true effectiveness [23].
In some other study, a group of patients treated with LITT for tough-to-control mesial temporal epilepsy, nearly all (97%) saw a reduction of at least 50% in seizure frequency compared to the start. Most (76%) experienced a reduction of at least 90%. In total, 83% of patients reached either a successful Engel Class I or II outcome. Specifically, 62% achieved Engel Class I, mostly with clear MTS. Meanwhile, 21% reached Class II, 17% Class III, and none reached Class IV [24], (Figure 2).
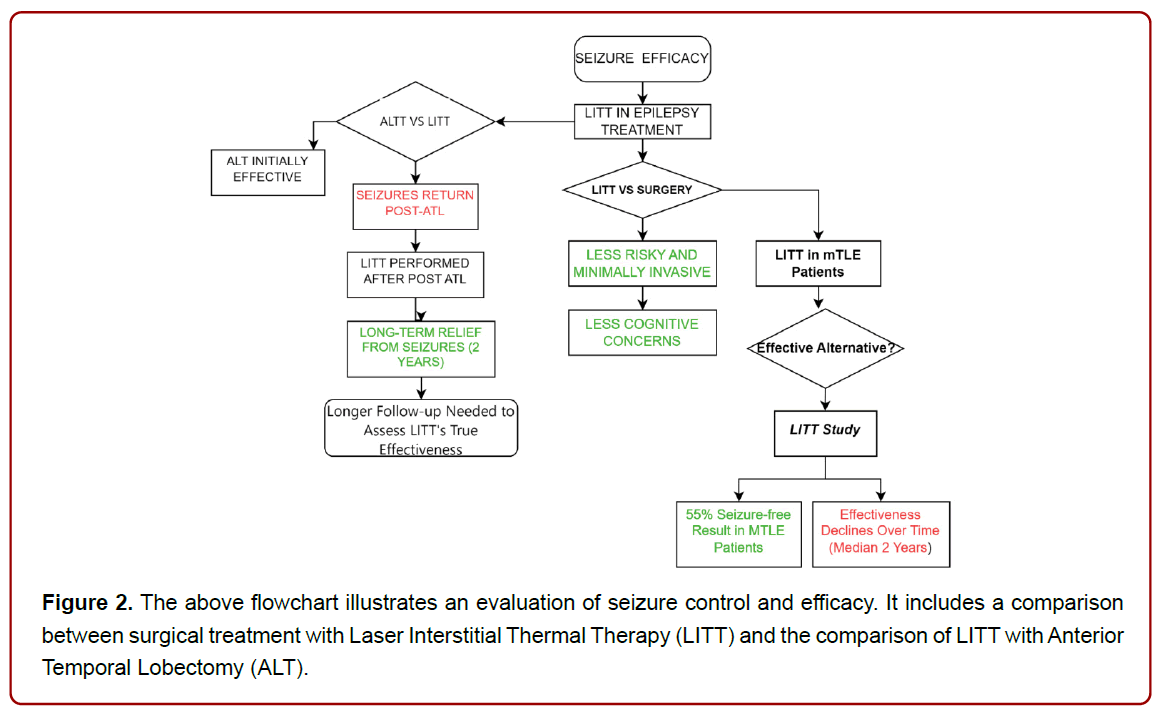
Figure 2. The above flowchart illustrates an evaluation of seizure control and efficacy. It includes a comparison between surgical treatment with Laser Interstitial Thermal Therapy (LITT) and the comparison of LITT with Anterior Temporal Lobectomy (ALT).
Safety and post-operative complications
While the advantages of LITT have been thoroughly documented, there appears to be an inadequate reporting of the associated complications. Post-operative complications include neurological complication, out of which the most frequently observed neurological morbidities include, visual field deficits take precedence, with reported occurrences spanning from 5% to 29% [25]. The prevalent adverse event post LITT, typically temporary, often manifests as contralateral superior quadrantanopia. This emergence of contralateral visual field deficits can be attributed to harm inflicted on the optic radiation at the posterior or the lateral geniculate nucleus [26]. Apart from that one study reported psychiatric complications, where 48% patients experienced a recurrence of their presurgical mood and/or anxiety episodes during the post-6-month period following their procedure [27].
Incorporating supplementary methodologies such as preoperative seizure localization through SEEG onset and functional mapping, intraoperative neuronavigation, and intraoperative MRI (iMRI) serves to enhance both safety and effectiveness, especially within the context of notable anatomical distortions following an LITT procedure [28].
While LITT constitutes a less intrusive technique the impact of a patient’s unique anatomy and the positioning of ablation areas influences the results in terms of seizures. The majority of treatment failures or successes linked to technical factors of the surgery arise from several reasons:
Laser trajectory, as it determines the ablation volume of the mesial temporal structures and safety profile of the procedure [29]. Optimal trajectories avoid sulci and CSF cavities and maximize distance from vasculature [30]. One research [31] revealed that trajectories situated closer to the inner portion of the hippocampal head exhibited a higher likelihood of being linked to an outcome of being free from seizures. Insufficient catheter depth to access the amygdala
Catheter placement is a critical aspect of ensuring proper function and minimizing complications to prevent issues such as infections, tissue damage, and incorrect fluid drainage. Complications associated with catheter placement include subdural hematoma [32] and arterial injury [33] and subarachnoid hemorrhage [34]. Precision in placement can be enhanced by using a stereotactic frame in conjunction with a metal skull anchor. This anchor is positioned above an alignment rod, passing through both the frame and the bolt. To minimize the potential for bleeding, the fusion of CTA and MRI can be employed to prevent vascular damage. Additionally, it is recommended to use a slender needle for dural penetration.
LITT hyperthermia; refers to an elevated body temperature that can result from various factors such as prolonged surgical procedures, infection, or brain injury which can negatively impact brain function, increase metabolic demands, and worsen tissue damage. LITT uses MRI-compatible fibers that transmit laser energy and are inserted into stereotactically placed cooling catheters to deliver targeted hyperthermia [35]. Reported complications from LITT hyperthermia itself include, new or worsening neurological deficits, diabetes insipidus [33], pseudoaneurysm [36], blood suffusion [37] edema requiring hemicraniectomy[33], and CSF leak [38]. Adjustments to hyperthermia levels are necessary when targeting ablation near vital neural structures like the brainstem, spinal cord, and eloquent cortex, especially when no fluid spaces are present in between. (Table 2)
| Complication Type | Description | PREVALANCE |
|---|---|---|
| Visual Field Deficits | Frequently observed post-operative neurological morbidity, often manifested as contralateral superior quadrantanopia. | Occurrence: 5% to 29% |
| Psychiatric Complications | Occurs post-6-month period following the procedure, including mood and/or anxiety episodes. | 48% experienced recurrence |
| Technical complications | Factors influencing treatment outcomes | |
| Improper Laser | Optimal trajectories avoid sulci and CSF cavities, Trajectory maximize distance from vasculature, and influence seizure outcomes | |
| Insufficient catheter depth | Critical for proper function, complications include subdural hematoma, arterial injury, subarachnoid haemorrhage. | |
| Catheter Placement | Precision using a stereotactic frame with a metal skull anchor; fusion of CTA and MAI for vascular damage prevention | |
| Complications from Hyperthermia | Complications include neurological deficits, diabetes insipidus, pseudoaneurysm, blood suffusion, oedema, CSF leak. |
Table 2. The table above provides an overview of complications that may occur after undergoing LITT surgery. It includes descriptions of these complications, their prevalence, and technical complications associated with the procedure.
Cognitive outcomes
The laser ablation method for treating MTLE is beneficial because it reduces cognitive issues, especially in the hemisphere responsible for language. While the results of MRI-guided laser therapy (MRIgLITT) aren’t as good as traditional surgeries, this is balanced by better cognitive function after surgery. Some decline in memory (specifically, CVLT–II short delayed cued recall and immediate memory from WMS–III Logical Memory subtest) and language abilities (Verbal IQ from WASI) was noted [39]. Temporary cognitive issues such as aphasia, stuttering, expressive dysphasia, and shortterm memory disturbances were observed as transient complications [40]. MRIgLITT serves as a proficient and impactful substitute for targeting the epileptogenic area and performing disconnection surgeries in individuals with stubborn epilepsy. This technique has the potential to notably enhance the management of seizures. MRIgLITT is linked to a relatively lower occurrence of complications, including transient neurological impairments [41]. LITT customized ablation maintains the integrity of white matter pathways leading to the lateral temporal regions. This preservation facilitates enhancements in memory scores and enables the incorporation of contextual information. This process leverages the capabilities of neighboring white matter tissue, which aids in the retrieval of information [42]. Successful LITT treatment may reduce the need for antiepileptic medications, potentially improving cognitive function without the negative effects of polypharmacy. (Figure 3) (Table 3)
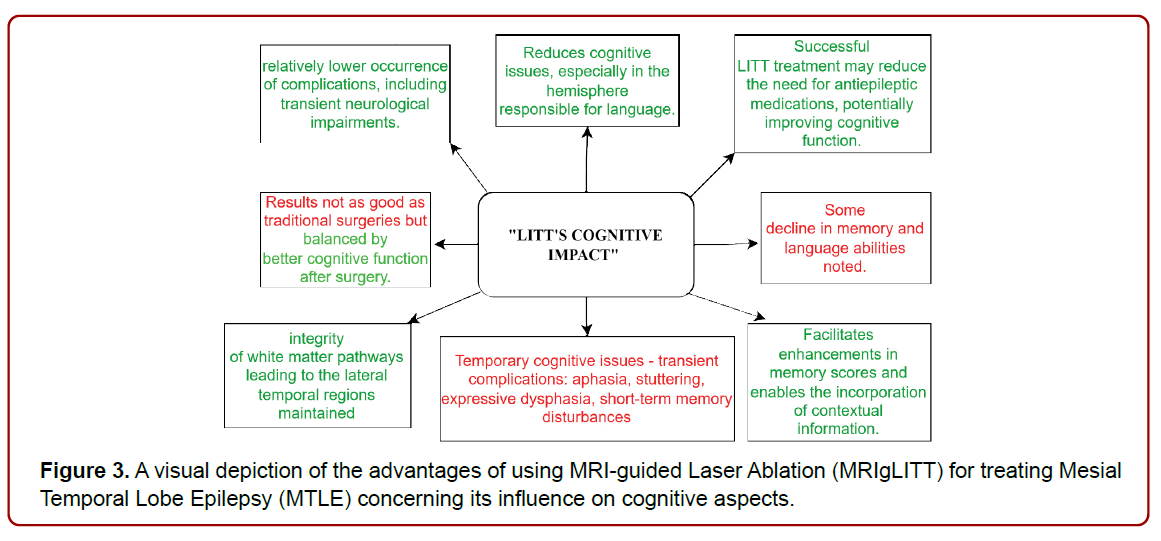
Figure 3. A visual depiction of the advantages of using MRI-guided Laser Ablation (MRIgLITT) for treating Mesial Temporal Lobe Epilepsy (MTLE) concerning its influence on cognitive aspects.
| Aspect | Benefits of MRI-guided Laser Ablation (MRIgLITT) for MTLE |
|---|---|
| Cognitive Function | Reduces cognitive issues, especially in the hemisphere responsible for language. |
| Surgery Results | Results not as good as traditional surgeries but balanced by better cognitive function after surgery. |
| Cognitive Decline | Some decline in memory and language abilities noted (e.g., CVLT–II short delayed cued recall, immediate memory from WMS–III Logical Memory subtest, Verbal IQ from WASI). |
| Transient Complications | Temporary cognitive issues observed as transient complications (e.g., aphasia, stuttering, expressive dysphasia, short-term memory disturbances). |
| Preservation of White Matter Pathways | LITT-customized ablation maintains the integrity of white matter pathways leading to the lateral temporal regions |
| Enhancements in Memory Scores | Facilitates enhancements in memory scores and enables the incorporation of contextual information. |
| Reduction in Antiepileptic Medications | Successful LITT treatment may reduce the need for antiepileptic medications, potentially improving cognitive function |
| Lower Occurrence of Complications | Linked to a relatively lower occurrence of complications, including transient neurological impairments |
Table 3. A tabular summary of Benefits of MRI-guided Laser Ablation (MRIgLITT) for MTLE
Quality of life and patient satisfaction
With the emergence of LITT as a less invasive surgical alternative to traditional craniectomy procedures for drug-resistant temporal lobe epilepsy, improved clinical outcomes have been observed in the form of shorter post-procedural hospital stays and reduced long-term neurocognitive side effects.
In 2012, a study published by Curry et al. [43] reported a groundbreaking result of a trial involving five pediatric patients with epileptogenic foci, showcasing the potential of LITT in achieving seizure freedom. Building on this foundation, Willie et al [32] embarked on a prospective case series involving thirteen patients undergoing fifteen LITT procedures for MTLE. The outcomes were promising, with a median hospitalization of just one day and notable percentages of patients achieving freedom from disabling seizures. The engagement of LITT in the treatment of MTLE, particularly in the context of MTS, underscored the potential for improved patient outcomes [32].
Furthermore, the significance of LITT’s impact on patient care becomes apparent when considering the clinical outcomes as demonstrated by several studies namely; Donos, 2018 [39], Landazuri, 2020 [44], Youngerman, 20205, Culler, 20226. Ablations targeting specific brain structures, including the amygdalohippocampal region, led to impressive Engel Class I outcomes [45]. Moreover, patient results were characterized by an astounding 76% achieving a greater than 90% reduction in baseline seizure frequency, highlighting the efficacy of the procedure. Importantly, none of the patients experienced worsening seizures (Engel Class IV outcome), accentuating the procedure’s safety profile [46].
Due to its minimally invasive nature, resulting in lower perioperative morbidity and shorter recovery times. Patients undergoing LITT reported minimal pain and discomfort, leading to shorter hospitalizations and quicker return to their daily lives. In addition, seizure-related worry and social functioning exhibited remarkable improvements in quality of life, signifying the broader impact of LITT on patients’ well-being [44]. (Figure 4)
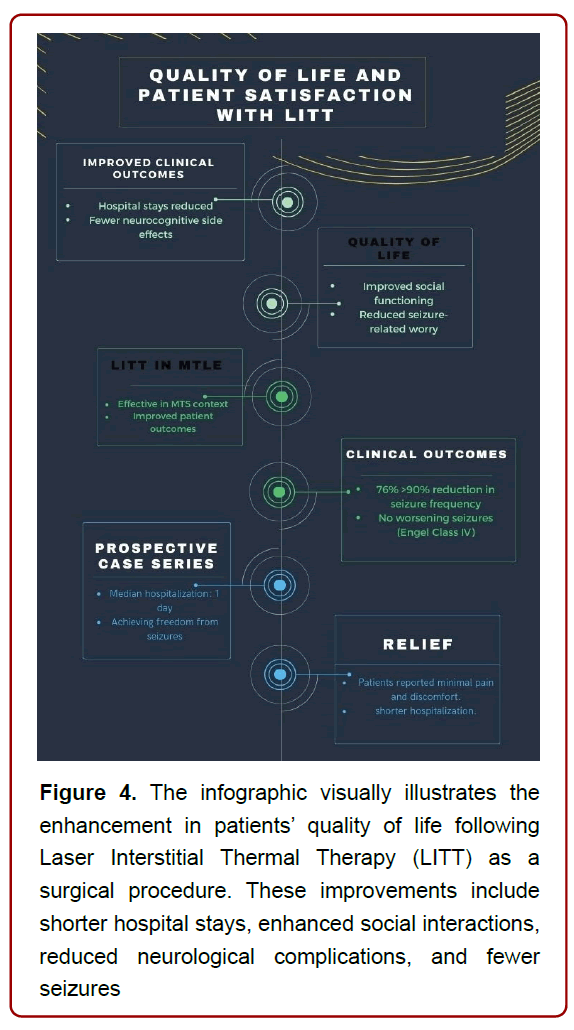
Figure 4. The infographic visually illustrates the enhancement in patients’ quality of life following Laser Interstitial Thermal Therapy (LITT) as a surgical procedure. These improvements include shorter hospital stays, enhanced social interactions, reduced neurological complications, and fewer seizures
Limitations and future directions
The nature of this communication restricts the inclusion of extensive data, potentially leading to a lack of detailed information regarding patient demographics, pre-existing conditions, and potential confounding factors. This limitation could result in less precise results. The study’s conclusions may not encompass potential variations in treatment response among diverse ethnic and cultural groups, which could potentially restrict the applicability of the findings. Due to space constraints, this brief communication may not fully incorporate perspectives from diverse disciplines like neurology, radiology, and neuropsychology, which could significantly enhance our overall comprehension of the treatment’s impact.
MRgLITT, as a minimally invasive avenue, demonstrates comparable efficacy, possibly motivating both epileptologists and patients to contemplate its application at an earlier stage of the disease, when concerns over invasive interventions might otherwise prevail. Present data indicates that MRgLITT is linked with relatively fewer complications, predominantly temporary neurological issues. Nevertheless, the absence of comprehensive largescale prospective studies complicates drawing definitive conclusions at present, so a need for of comprehensive large-scale prospective studies come into view [47]. Additional challenges encompass the absence of a consensus regarding the optimal dosage of thermal energy per unit volume for precise tissue ablation [48]. Moreover, a lack of established surgical protocols or standardized workflows poses an additional obstacle [48]. These lacking point us in the direction which we need to take in the future to make clinical application of LITT more feasible which is to establish better protocols for clinical application of LITT via research which will also solve the issue of determining optimal dosage of thermal energy per unit volume for precise tissue ablation. We hold the view that MRgLITT holds a promising trajectory as a standalone treatment or when employed in conjunction with conventional open surgery. Prospective trials focusing on safety aspects and the establishment of a standard protocol constitute critical avenues for future research.
In the realm of epilepsy treatment, an exciting paradigm shift has emerged through the advent of Laser Interstitial Thermal Therapy (LITT), also known as Stereotactic Laser Ablation (SLA). This minimally invasive technique presents a groundbreaking alternative to traditional open surgery, offering a transformative approach for eradicating epileptogenic zones, intracranial tumors nestled in deepseated regions, and recurrent metastases [49-51]. At the one-year follow-up mark, a notable 53% of all patients showcased enhanced seizure frequency (Engel Class I or II), underscoring the technique’s versatility. Published reports underscore the transformative potential of LITT [39,52], heralding it as a less invasive surgical solution with shorter hospital stays and diminished neurocognitive side effects compared to conventional craniotomy approaches. In conclusion, the trajectory of LITT’s success is apparent, holding the promise to redefine the landscape of epilepsy treatment through improved patient outcomes, enhanced quality of life, and a brighter future for those previously burdened by the complexities of drug-resistant epilepsy.