Ravi Kumar Chittoria 1*,Kanav Gupta2,Nagarjun S Ghatti3
1 Senior Professor and Associate Dean (Academic), Head of IT Wing and Telemedicine Department of Plastic Surgery and Telemedicine JIPMER, Puducherry, India
2Senior Resident Department of Plastic Surgery JIPMER Puducherry, India
3Observer, Department of Plastic Surgery JIPMER Puducherry, India
Received: 18 Nov 2025; Accepted: 05 Dec 2025; Published: 16 Dec 2025
Citation: Ravi Kumar Chittoria, Kanav Gupta, Nagarjun S Ghatti. “Role of Cyclical Negative Pressure Wound Therapy in Pressure Sore Grade IV.” J Aesthetic Surg Med (2025): 110. DOI: 10.59462/3068-5311.2.1.110
Copyright: © 2025 Ravi Kumar Chittoria..This is an open-access arti cle distributed under the terms of the Creative Com mons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The application of “negative pressure” has evolved to a cornerstone in the treatment of acute and chronic wounds in almost all specialties. Continuous Intermittent, cyclic are the three types of Negative pressure Wound Therapy (NPWT). The cyclic NPWT system is similar to the intermittent mode in terms of using the same maximal sub atmospheric pressure, but the pressure never reaches zero in the cyclic mode. Cyclic application of “negative pressure” results in a superior local enhancement of cutaneous microcirculation with regards to blood flow and consecutive tissue oxygenation. In this article, cyclic NPWT was compared with other NPWT.
Negative Pressure Wound Therapy, Cyclic, Wound.
Since the introduction of the negative pressure wound
therapy (NPWT) system by Morykwas and Argenta,
it has been applied to a number of wounds and has
become an influential and effective technique for healing simple and complex wounds. The conventional
NPWT system adopts either ‘intermittent’ or ‘continuous’ mode.
While the continuous mode constantly applies a
sub-atmospheric pressure of −125 mmHg, the intermittent mode creates a sub-atmospheric pressure
of −125 mmHg for 5 minutes and a 2-minute resting
phase of 0 mmHg.
In experiments performed on animal models, the intermittent mode showed increased perfusion
level
and formation of granulation tissue in the wound area
compared with the continuous mode. [1,2] Despite the
effectiveness of intermittent mode in wound healing,
it has been avoided in clinical application because of
the pain occurring every few minutes during the initiation phase of the system to reach −125 mmHg. Thus,
‘cyclic’ mode would minimize the pain while maintaining the superior efficacy of the intermittent mode.
The cyclic NPWT system is similar to the intermittent
mode in terms of using the same maximal sub at mospheric pressure, but the pressure never reaches
zero in the cyclic mode. So, it continuously creates
certain pressure gradient that oscillates between −125
mmHg and the preset sub atmospheric pressure. The
cycle runs based on the changes in sub atmospheric
pressure, not time, and thus its frequency reflects the
wound volume.
The study is done in a tertiary care hospital in South India. The subject is A 55-year-old female known diabetic uncontrolled and parotid carcinoma with an alleged history of lower limb weakness with incontinence of stools and urine. She developed ulcers in the sacral and greater trochanter regions (Fig 1). The patient had undergone multiple dressings of functional regenerative therapy with scaffold and cyclical NPWT post debridement (Fig 2). Cyclical NPWT five applications were done.
Fig 1:wound time of admission
Results
Cyclical NPWT is useful in improving the wound healing of pressure sore in patient as we have seen in this
study (Fig 3,4).
Fig 2:after applying CRONPWT
Results
Cyclical NPWT is useful in improving the wound healing of pressure sore in patient as we have seen in this
study (Fig 3,4).
Fig 3:Wound after the CRONPWT Treatment
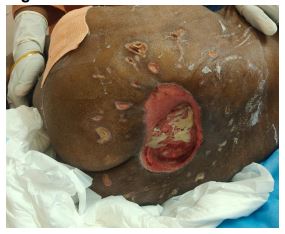
Fig 4:Wound after the CRONPWT Treatment
Over the past decades, the application of “negative pressure” has evolved to a cornerstone in the treatment of acute and chronic wounds in almost all specialties. Various available synonyms reflect the past developments and current applications of the technique involving, among others, “Vacuum-assisted closure” (VAC), “Negative Pressure Wound Therapy” (NPWT), “closed incision Negative Pressure Therapy” (ciNPT), or “Negative Pressure Wound Therapy with instillation” (NPWTi). [3] All but ciNPT are used for treatment of open wounds and exert the known beneficial effects of “negative pressure” therapy on wound healing, i.e., sufficient temporary wound closure, promotion of wound bed granulation, mechanical contraction and stabilization of wound margins, and efficient reduction of bacterial load. Wound bed perfusion represents another key factor in wound healing. Effects of “negative pressure” on wound bed perfusion have lately been widely discussed.
Within this preclinical study on acute changes of cutaneous microcirculation under an applied NPWT
dressing, we observed a significant increase of local
perfusion dynamics with consecutive improvement of
tissue oxygen saturation.
Interestingly, all three compared modes of application,
continuous, intermittent, and cyclic, resulted in locally
enhanced microcirculation of a greater or lesser extent.
In the comparison of different application modes, we
observed superior effects on local and remote cutaneous perfusion in the cyclic group.
The continuous mode
represented the most common
setting in clinical wound care according to a published
meta-analysis [11], in which discontinuous applications were rarely reported. [11]
Notably, continuous treatment
represents the generally accepted standard of care despite already available early evidence of superior capabilities
of an intermittent NPWT treatment with respect to formation
of granulation tissue or angiogenesis. Most likely, this
is attributable to the fact that intermittent activation of
“negative-pressure,” which causes repeated spikes in
surface pressure to the wound, is believed to be unpleasing.
Lately, the introduction of the “cyclic-mode”
appears
as a promising compromise combining both the satisfaction of patients and superior wound treatment. [12]
Pain levels were generally low in cyclic NPWT.
In human cutaneous microcirculation, resting capillary pressure
was reported in a range from 10.5 to
22.5 mmHg or even 41.0 mmHg [13,14]. Thus, applied
surface pressure of ~30.0 mmHg via a NPWT dressing could potentially result in an occlusion of cutaneous
capillaries. Given the finding that capillary pressure also increases in response to a higher venous
pressure, at least a sub-total occlusion of the dermal
microvasculature due to the intervention can be assumed. [15] Overall, the mechanisms of cutaneous
vascular response to certain stimuli are complex, especially concerning vasodilation and improvement of
local flow. [16] Repeated capillary (subtotal) occlusion
represents a strong stimulus for the affected tissue.
Both post-occlusive reactive hyperemia (PORHA) and
increased mechano-humoral transduction to the vascular bed result in alterations of intravascular shear
stress and could be accountable for superior effects
in the intermittent and, particularly, in the cyclic group.
[17,18] We also assessed changes of cutaneous microcirculation on the contralateral thigh and found
stronger effects in the cyclic group. Previous studies
on Remote Ischemic Conditioning (RIC), showed alterations in the applied stimulus can influence the triggered
improvement of cutaneous perfusion. [19,20]
Duration of applied pressure, number of repeated cycles, and body site are important variables to optimize
the conditioning effect on the improvement of remote
microcirculation.
Cutaneous capillary network can be investigated with regards to blood flow (BF), velocity (VELO), postcapillary oxygen saturation (StO2), and relative hemoglobin content (rHb). [11]
Regardless of the application of different pressure levels, intervals of suction and cutaneous blood flow below the foam dressing was significantly enhanced in all three types.
Corresponding to enhancements in cutaneous BF, StO2-values steadily increased when suction was active.
Both parameters were significantly altered due to the NPWT stimulus.
As expected, reported levels of discomfort were nominal. No statistic difference was found in comparison of maximum values between groups (p > 0.05).
Applied suction caused significant changes in the surface pressure (sp) of the underlying skin.
Cutaneous microcirculation of the contralateral thigh was also affected by NPWT treatment. It shows virtually a linear increase in BF 90 min in all three types.
Cyclic application of “negative pressure” results in a superior local enhancement of cutaneous microcirculation with regards to blood flow and consecutive tissue oxygenation. Beyond that, repeated alterations between different levels of “negative pressure” due to cyclic application represent a greater stimulus for remote conditioning effects, indicating a superior local interaction with the underlying tissue. Further research is warranted to investigate the correlation between local perfusion enhancements and granulation tissue formation due to cyclic NPWT in humans.