Strategies to Adopt in a Challenging Laparoscopic Cholecystectomy
Joshua Sungho Hong 1,2*,Radha Nair1
1Swan Hill District Health, Swan Hill, Victoria, Australia
2St. Vincent’s Hospital Melbourne, Victoria, Australia
*Corresponding Author:
Joshua Sungho Hong,
Hill District Health,
Swan Hill, Victoria,Victoria, St. Vincent’s Hospital Melbourne, Victoria, Australia.
E-mail: joshua.hongsh@gmail.com
Received: 223 May 2025; Accepted: 16 June 2025; Published: 23 June
2025
Citation: Joshua Sungho Hong,Nair R. “Strategies to Adopt in a Challenging Laparoscopic
Cholecystectomy” J Aesthetic
Surg Med (2025): 108. DOI: 10.59462/
JASM.2.1.108
Copyright: © 2025 Joshua
Sungho Hong. This is an open-access arti cle distributed under the terms of the Creative Com mons
Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
Abstract
Laparoscopic Cholecystectomy [LC] is among the
most performed surgical procedures worldwide.
While typically straightforward, a subset of cases
presents significant technical difficulty due to
inflammation, fibrosis, or anatomical distortionfactors that dramatically increase the risk of bile
duct injury [BDI], conversion to open surgery, and
other complications. Risk factors of a challenging
cholecystectomy are male sex, older age,
obesity, acute or recurrent cholecystitis, cirrhosis,
and anatomical anomalies. A strong emphasis
is placed on hepatobiliary anatomy, including
variants of the cystic duct, cystic artery, Rouviere’s sulcus, and accessory bile
ducts, which play a critical role in surgical safety.
Mirizzi’s syndrome, though rare, represents a
particularly hazardous scenario and is discussed
in detail with respect to its classification and
operative implications. Core surgical strategies
are reviewed, highlighting the Critical View
of Safety [CVS], liberal use of intraoperative
cholangiography, and the value of intraoperative
pauses. Bailout techniques, including subtotal
cholecystectomy, fundus-first dissection, and
conversion to open surgery, are presented as
essential tools to mitigate risk when standard
dissection is unsafe or unachievable. Subtotal
cholecystectomy is recognised as a key damagecontrol option with excellent outcomes in expert
hands. Emerging adjuncts such as Indocyanine
Green [ICG] fluorescence cholangiography,
robotic surgery, and early artificial intelligence
technologies are explored for their roles in
enhancing intraoperative visualisation and
decision-making.
Ultimately, safe management of the difficult
gallbladder requires anatomical expertise, a
culture of intraoperative vigilance, and readiness
to adapt when standard approaches are
inadequate. This review aims to equip surgeons
with a structured framework to anticipate difficulty,
avoid injury, and optimise outcomes in complex
cholecystectomy scenarios.
Keywords
Laparoscopic Cholecystectomy; gallbladder; Mirizzi’s syndrome; subtotal cholecystectomy; Anatomy
Introduction
Laparoscopic Cholecystectomy [LC] is one of the most
common operations performed worldwide, with hundreds of thousands done annually [1]. Laparoscopic
Cholecystectomy [LC] has become the gold-standard
for treating symptomatic gallstone disease, resulting
in reduced post-operative pain, orbidity, and faster
recovery compared to open surgery [2]. Despite the
routine nature of LC, a subset of cases are deemed
“difficult” or challenging, posing significant clinical importance [3]. A difficult LC is associated with longer
operative times, higher conversion to open surgery, increased complication rates [including bile duct injury],
and extended hospital stay. Difficult LC are generally classified as the ones with severe inflammation or
scarring distorts the biliary anatomy and complicates dissection.
Situations like acute calculous cholecystitis
[especially if gangrenous or emphysematous], gallbladder perforation, Mirizzi syndrome [an impacted stone causing
biliary compression or fistula], or chronic fibrosis
can obscure Calot’s triangle and make identification
of structures challenging [4]. In one large review
[>300,000 patients], factors like acute or chronic
cholecystitis, “bad gallbladder” pathology [necrosis,
gangrene, etc.], cystic duct stones, anatomical variants, and cholecystoenteric fistulas were all linked to
difficult cholecystectomy [5]. Risk factors associated
with a difficult LC includes male sex, age, acute on
chronic cholecystitis, obesity and cirrhosis of the liver
[6-8]. LC is the leading cause of Bile Duct Injury [BDI]
in surgery, and the vast majority of major BDIs occur
in these difficult scenarios with severe inflammation or
aberrant anatomy [9]. BDIs can have significant morbidity impacts on patients. Therefore, understanding
how to predict, prepare for, and manage a challenging cholecystectomy is of a paramount clinical significance. This
paper provides a comprehensive review
of difficult cholecystectomy management-including
pertinent anatomy, evidence-based guidelines, surgical techniques, bail-out strategies, and emerging
technologies.
Hepatobiliary Anatomy and Common Variations
A thorough knowledge of the hepatobiliary anatomy
and its many variations is critical for safely navigating a difficult cholecystectomy. Key anatomic relationships in
the hepatocystic [Calot’s] triangle are paramount. The classic Calot’s triangle is bounded by the
cystic duct, common hepatic duct, and inferior surface
of the liver; it typically contains the cystic artery, lymph
node of Lund, and fatty areolar tissue [10]. In reality,
the upper boundary is the liver, and surgeons refer to
the “Calot’s” or hepatocystic triangle as the space that
must be cleared to achieve the critical view of safety
[11]. Inflammation [acute or chronic] can fill this triangle with fibrous tissue and adhesions, obscuring the
normal landmarks [12]. Scarring may adhere the gallbladder to surrounding structures, making the cystic
duct and artery difficult to identify. Important anatomical structures and variants to consider include:
Calot’s Triangle & Critical View
Proper identification of the cystic duct and cystic artery within Calot’s triangle is paramount. The “Critical
View of Safety” [CVS] technique entails clearing all fat
and fibrous tissue from Calot’s triangle, exposing the
cystic duct and artery entering the gallbladder, and
confirming the gallbladder is separated from the liver bed except at the cystic structures [13]. In difficult cases,
achieving the CVS can be challenging but remains the gold standard for avoiding misidentification
injuries. An inflamed lymph node of Lund or dense adhesions may be encountered and should be carefully
dissected to reveal the cystic artery beneath [11].
Rouviere’s Sulcus
Rouviere’s sulcus is a natural fissure on the liver’s
undersurface which is present in ~80% of individuals
that corresponds to the plane of the right porta hepatis
[14]. It runs to the right of the hepatic hilum and, when
visible, serves as a safety landmark during cholecystectomy. A general rule is to keep dissection above the
level of Rouviere’s sulcus to avoid injuring the common bile duct which lies below that plane. Drawing an
imaginary line from the sulcus to the umbilical fissure
[the R4U line] demarcates a safe zone for dissection
on the gallbladder versus the danger zone deeper toward the porta hepatis [12]. In a hostile anatomy scenario,
identifying Rouviere’s sulcus can help orient the
surgeon when usual landmarks are lost.
Cystic Artery and Variants
In about 70-80% of cases, the cystic artery arises from
the right hepatic artery and traverses within Calot’s triangle [15]. Commonly it bifurcates into superficial and
deep branches to the gallbladder. A notorious variation is a short cystic artery arising from a tortuous
right hepatic artery that loops [sometimes termed a
Moynihan’s hump or caterpillar turn]. The right hepatic
artery may be mistaken for the cystic artery because
it gives off a very short cystic branch. A catastrophic
injury could arise if one erroneously clips the right hepatic artery [16]. Therefore, caution should be taken
when the “cystic artery” seems unusually large or low
in the field, as it could potentially be an aberrant or
early-branching right hepatic artery [17].
An accessory cystic artery might arise from the gastroduodenal artery
or left hepatic artery. The cystic
artery usually passes behind the cystic duct [retroductal], but it can run anterior to the duct in around 4–5%
of cases [18].
Bile Duct Variations
There are numerous biliary tree anomalies that can
make a cholecystectomy treacherous. An aberrant
right sectoral duct [often the right posterior sectorial
duct] can sometimes empty directly into the gallbladder or cystic duct instead of the common hepatic duct.
Such a duct can be easily misidentified as the cystic
duct and mistakenly divided [19].
An
important anomaly is the presence of accessory or
aberrant bile ducts. Small subvesical bile ducts [often
called ducts of Luschka] can connect liver segments
directly to the gallbladder bed; minor ones are present
in up to 12–50% of people [20]. While they usually are tiny (<1– 2 mm) and not seen intraoperatively, if
one is relatively large and gets clipped or damaged, it
can cause a postoperative bile leak. About 0.2–2% of
cholecystectomies incur bile leaks, often from a cystic
duct stump or an unrecognised duct of Luschka [21,
22]. Indeed, the most common mechanism of major
bile duct injury is misidentification-e.g. confusing the
common bile duct or an accessory duct for the cystic
duct in an inflamed field. Low insertion of the cystic
duct or a parallel course of the cystic duct can also
cause inadvertent cut into the common duct. Routine
use of landmarks like the Rouviere’s sulcus and techniques like intraoperative cholangiography or intraoperative
usages of Indocyanine Green (ICG) can help
in better visualisation of the ductal anatomy thus allowing identification of anomalies.
Mirizzi’s Syndrome
Mirizzi’s syndrome is a rare complication of gallstone
disease where a stone becomes impacted in the cystic
duct or Hartmann’s pouch of the gallbladder and compresses the common hepatic duct. Chronic compression can lead to
erosion and formation of a cholecysto-biliary fistula between the gallbladder and common
bile duct. Mirizzi syndrome is uncommon (occurring
less than 1% in Western countries) [23]. Its presence
dramatically increases the risk of bile duct injury if unrecognised and has a reported bile duct injury rates up
to 17% when Mirizzi is undiagnosed at surgery [24].
Preoperative diagnosis can be challenging because
the presentation can mimic ordinary choledocholithiasis or cholecystitis, thus identified in only 8–62% of
cases [25]. Therefore, it is crucial to have a high index
of suspicion in patients with long-standing gallstones
who present with obstructive jaundice, cholangitis or
a shrunken gallbladder with an impacted stone in the
Hartmann’s pouch on imaging.
The Csendes classification is the most widely used
system to categorize Mirizzi syndrome and is based
on the presence and extent of cholecysto-biliary fistula and any cholecysto- enteric fistula [Figure 1] [26].
-
Type I: External compression of the common hepatic duct by an impacted stone in the cystic duct
or infundibulum, without any fistula. The bile duct lumen is narrowed by extrinsic pressure, but its wall is
intact.
-
Type II:A cholecysto-biliary fistula is present, with
erosion of the gallstone from the gallbladder into
the bile duct, involving less than one-third of the
bile duct circumference. There is a presence of a
small defect between the gallbladder and common
bile duct.
-
Type III:A larger cholecysto-biliary fistula involving roughly up to two-third of the bile duct
circumference. A substantial portion of the duct wall is
destroyed by the inflammatory erosion, creating a
sizable communication.
-
Type IV:A complete cholecysto-choledochal fistula replacing the common duct, involving 100% of
the bile duct circumference. The gallbladder and
bile duct form one confluent cavity due to total wall
destruction.
-
Type V:Mirizzi’s syndrome with a cholecysto-enteric fistula [a bilio-digestive fistula to
nearby viscus]. This typically results from the same inflammatory process extending to the duodenum or
colon.
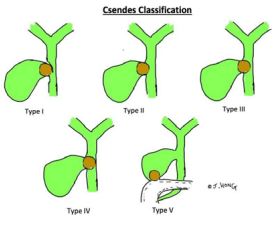
Figure 1.Diagrammatic representation of Csendes Classification
Types I and II are the most common presentations,
whereas the higher types are relatively rare [23]. Mirizzi’s syndrome is usually diagnosed on imaging such
as MRCP, CT scan or ultrasound. Mirizzi’s syndrome
should be suspected if the patient has obstructive
jaundiceplus gallstones without a clear common duct
stone, or if imaging shows a stone in an infundibular
position with bile duct dilation upstream. Intraoperatively, finding a gallstone eroding into the duct or an
unusually large inflammatory mass at Calot’s triangle
signals a Mirizzi’s.
Management depends on the type: For Type I [external compression without fistula], a careful subtotal
cholecystectomy or even cholecystectomy can often
be done, removing the gallstone and gallbladder while
avoiding injury to the bile duct, sometimes combined
with a cholangiogram to confirm duct patency. For
Types II–IV [fistulas], the operation can involve a bile
duct repair. Small fistulas may be managed with choledochoplasty using a flap of cystic duct or gallbladder remnant
to close the defect. Some has proposed
a self-expanding metal stent via ERCP for small bile
duct defects [27, 28]. Larger defects might require
biliary reconstruction such as a hepaticojejunostomy.
Because the tissue is inflamed and scarred, these are
high-risk cases for bile duct injury or stricture. Some authors recommend open surgery for higher-grade
Mirizzi’s syndrome, or at least a very low threshold
to convert from laparoscopy to open, due to the advanced dissection and suturing often required. If a
cholecystoenteric fistula (Type V) is present, one must
also address the intestinal fistula and be vigilant for
gallstone ileus in the bowel.
Knowing the classifications helps in anticipating the
required surgical steps and risks. Crucially, if Mirizzi
syndrome is encountered or suspected, subtotal cholecystectomy could be performed, identification of the
bile duct and fistula extent, and often performing an
intraoperative cholangiogram or using choledochoscopy to evaluate the common duct. A difficult Mirizzi’s
case should prompt consideration of involving hepatobiliary services for ongoing management.
Strategies and Best Practices for Difficult Laparoscopic Cholecystectom
Safe technique and a systematic approach are crucial
when performing a cholecystectomy in difficult conditions. The primary goal is to avoid bile duct or vascular
injuries while successfully removing the gallbladder. A
“culture of safety” in cholecystectomy has been promoted by surgical societies [e.g. SAGES Safe Cholecystectomy
program] to systematize the practice of
safe techniques.
- Identifying and aiming to achieve the Critical View
of Safety [CVS] before dividing any duct or artery
is paramount. To attain the CVS, three criteria
must be met: [a] the hepatocystic [Calot’s] triangle
is cleared of all fat and fibrous tissue, [b] the lower third of the gallbladder is dissected off the liver
bed to expose the cystic plate, and [c] only two
structures (the cystic duct and cystic artery) are
seen entering the gallbladder, with no other connections. This creates a “funnel” or “tent” appearance
confirming the anatomy [29]. Most bile duct
injuries occur when CVS was not attained and the
wrong duct is ligated [30]. The efficacy of CVS is
well-supported: for example, one large series of
1,046 laparoscopic cholecystectomies [998 done
with CVS technique] reported zero major bile duct
injuries and only 5 minor bile leaks, with a low conversion rate of 2.7% [31].
- In any cholecystectomy, anatomical anomalies
should be considered. For example, an absent
cystic duct (cystic duct fused with the gallbladder)
or a short cystic duct can occur in chronic cholecystitis; an accessory bile duct might be draining from
the right lobe into the gallbladder; the right hepatic
artery might be anomalous. Being conscious of an
aberrant duct or vessel, it will increase vigilance to
double-check anatomy before ligation.
- A low threshold for obtaining an intraoperative
cholangiogram is advisable in difficult cases [5].
Cholangiography can opacify the biliary tree to
delineate the cystic duct/common bile duct junction and identify any stones or abnormal ducts.
Routine or selective IOC remains controversial,
but evidence suggests it can both detect common
bile duct stones and potentially alert the surgeon
to misidentification before an irreversible error is
made. Alternatives or adjuncts to IOC include laparoscopic ultrasonography or newer techniques
like near-infrared fluorescent cholangiography
with Indocyanine Green [ICG].
- An additional safety step advocated in recent
guidelines is an intraoperative pause – double
checking after dissection and identification of critical structures before ligating. This can include
having the assistant confirm the view, ensuring the
gallbladder is dissected off the liver; double confirming the CVS. Only when everyone is satisfied
should clipping and division proceed.
- Recognising the “Inflection Point” is the most important judgment in a difficult cholecystectomy.
Strasberg described an “inflection point” as the
moment where safely obtaining the critical view
is not possible, and that continuing could lead to
injury [32]. Inability to define structures after significant effort due to dense fibrosis obscuring tissue
planes [frozen Calot’s triangle], severe inflammation with purulence, or finding oneself disoriented
about the anatomy. Further dissection in Calot’s
triangle should be stopped and convert to a bailout strategy (e.g. subtotal cholecystectomy, conversion to open,
placing a cholecystostomy tube).
- Seeking help from a second experienced surgeon to assist or take over if things are extremely
challenging. A fresh perspective, or another set
of skilled hands, can sometimes navigate a tricky
dissection more easily. Referral to a hepatobiliary
service should be made early when it is beyond
the scope of one’s capabilities.
In summary, the best practices for difficult cholecystectomy center around strict adherence to safe dissection
principles, excellent visualization, liberal use
of intraoperative imaging, and the courage to halt and
choose a safer alternative when anatomy is uncertain.
Bailout Strategies for the Difficult Gallbladder
Bailout strategies are considered when conventional
dissection cannot be safely performed to avoid catastrophic injury. These techniques aim to resolve the
acute problem [remove the septic gallbladder or decompress it] while avoiding blind dissection in dangerous areas.
The main bailout options include subtotal cholecystectomy, the fundus-first [dome-down] technique, and conversion to
open surgery. In extreme
cases, aborting the cholecystectomy and performing a
cholecystostomy [drainage of the gallbladder] may be
the safest approach. Each strategy is chosen based
on intraoperative findings and the operator’s judgment.
Subtotal Cholecystectomy
Subtotal Cholecystectomy (STC) involves intentionally not removing the entire gallbladder. Indications for
STC are a hostile Calot’s triangle where the cystic duct
and artery cannot be safely isolated due to inflammation or fibrosis. The gallbladder can be truncated at
the body/fundus and leaving the portion of gallbladder
neck/cystic duct in situ which will markedly reduce the
risk of tearing the common bile duct or hepatic artery.
Two main variants are described:
- Fenestrating STC: The gallbladder is opened and
the stones and contents evacuated, and usually
the back wall of the gallbladder is left at the gallbladder fossa. The cystic duct may be left open or
suture-ligated from inside if reachable.
- Reconstituting STC: The remnant gallbladder
neck is closed by suture or stapler, reconstituting
a small “gallbladder stump” that remains. This resembles actually finishing the cholecystectomy
except that a portion of the gallbladder wall is left
attached to the cystic duct, which is closed. [33].
Both approaches avoid dissecting in the hazardous
area of the porta hepatis and a drain should be left
in-situ before bailing out.
Subtotal cholecystectomy has become one of the
most important bailouts in difficult cholecystectomy.
Evidence shows that STC dramatically lowers the risk
of bile duct injury compared to persisting with a total
cholecystectomy in severe inflammation [34]. It can
almost always be performed laparoscopically, thus
obviating the need for a large open incision in many
cases. Recent literature suggests that subtotal cholecystectomy is the single most effective bailout procedure currently
for difficult gallbladders [33]. A 2016
HPB analysis succinctly concluded that the morbidity
from bile duct injury far exceeds the morbidity from
a subtotal cholecystectomy, firmly establishing STC
as a reasonable and safe bailout strategy [35]. Bile
leakage from the stump is a known complication, especially with the fenestrating technique, so a drain is
typically left and the cystic duct may be secured with
a suture or endoloop if possible. Patients should be
informed that a remnant gallbladder remains, which in
rare cases can cause a remnant cholecystitis. However, long- term follow-up of subtotal cholecystectomy patients is
generally positive: the vast majority [over90%] have symptom resolution and avoid re-operation [36].
Fundus-First [Dome-Down] Approach
The fundus-first technique [also called dome-down
cholecystectomy] is both a strategy for difficult cases
and a potential step in a bailout. The gallbladder is dissected from the liver bed at the fundus and dissected
down towards the cystic duct. This allows early identification of the cystic duct and artery when extensive
scarring is present at Calot’s triangle. The fundus-first
dissection could allow gallbladder to be dissected except for a small stump, intentionally converting the situation
into a subtotal cholecystectomy. Key step is to
stay in the correct tissue plane during dissection – the
plane between the gallbladder serosa and the cystic
plate of the liver. Large vessels of the right portal pedicle or a hole into the common duct from the side could
be created if dissection goes into liver parenchyma or
too deep toward the hilum.
In many difficult cholecystectomies, fundus-first approach is often used in tandem
with subtotal cholecystectomy. Thus, fundus-first is an important tool in
the arsenal and can be considered a form of “bailout”
itself – an alternative dissection route that avoids
the most inflamed area initially [37]. In summary, the
dome-down technique is a valuable strategy in difficult
gallbladders, often leading to a controlled subtotal resection depending on the intra-operative assessment.
Conversion to Open Surgery
Conversion to an open cholecystectomy remains an
ever-present bailout option and is indeed the original
fail-safe plan in laparoscopic surgery. Every Laparoscopic Cholecystectomy carries a risk of conversion [a
few percent in elective cases, but significantly higher
in acute or difficult cases]. Hesitation should not be
made where laparoscopy does not allow safe progress due to severe inflammation, unclear anatomy, excessive bleeding,
or other technical impasses.
Studies have shown that conversion rates in difficult
cholecystectomy vary widely depending on the threshold of the surgeon and the patient population. Experienced
minimally invasive surgeons, using bailouts like
subtotal resection, have reported very low conversion
rates [38]. The introduction of bailout techniques like
laparoscopic subtotal cholecystectomy has reduced
the need for open conversion in many centers, since
the surgeon can avoid the dangerous dissection without needing to abandon laparoscopy [39]. Conversion
to open is only beneficial if the procedure can be completed safely. Therefore, it is recommended that every
surgeon should be adept at open biliary surgery or
have a colleague available who is. If a surgeon is not
comfortable with complex open biliary dissection [for
example, a laparoscopy-trained surgeon who rarely does open cases], involvement of an HPB (hepatopancreatobiliary)
specialist is wise either intraoperatively
or by early referral.
Recent Advances and Adjuncts in Difficult Cholecystectomy
Modern technology and evolving techniques are continually improving the safety of cholecystectomy, especially in
difficult scenarios. Here we discuss a few
recent advances and adjuncts that are particularly
relevant: robotic-assisted cholecystectomy, routine or
selective intraoperative cholangiography, Indocyanine
Green (ICG) fluorescence guidance and artificial intelligence.
- 1. Robotic-Assisted Cholecystectomy:
Robotic surgical systems have been increasingly applied
to cholecystectomy in the recent years. The robotic platform offers high-definition 3D visualisation
and wristed instruments that enhance dexterity compared to straight laparoscopy, which could be advantageous in
dense adhesions or awkward anatomies.
Some reports suggest that robotic cholecystectomy
can overcome difficulties related to visualisation and
instrument maneuverability in difficult gallbladders,
potentially reducing conversion rates in acute cholecystitis cases. For example, a recent study in an
emergency setting found robotic cholecystectomy had
a significantly lower risk of conversion to open surgery compared to laparoscopic in similar patients [40].
However, the data on improved outcomes with robotics are mixed. Smaller series have found the robotic approach
safe and feasible in complex cases, but
a large analysis of Medicare data reported a higher
incidence of bile duct injury with robotic cholecystectomy as compared to standard Laparoscopic Cholecystectomy
[41, 42]. This might reflect the learning
curve and early adoption phase; as robotics becomes
more widespread, outcomes may improve. At present, robotic cholecystectomy is generally considered
as effective as laparoscopy for gallbladder disease,
with the potential for improved surgeon ergonomics
and precision in difficult cases. The downsides include
higher cost, longer setup time, and limited availability
in many centers. The consensus is that robotics is a
promising adjunct for difficult cholecystectomy but not
an absolute necessity.
The decision to use robotics often comes down to
surgeon preference and resource availability. robotic cholecystectomy is an emerging tool that can be
considered in difficult cases, showing some trend
toward lower conversion, but its impact on bile duct
injury rates is not clearly proven and vigilance is still
required.
- Intraoperative Cholangiography [IOC]:
Intraoperative cholangiography is a classic adjunct
rather than a new one, but it continues to be a topic of
debate and improvement in the context of difficult cholecystectomy. IOC involves cannulating the cystic duct
and injecting contrast to visualize the biliary tree under
fluoroscopy during surgery. Its utility in difficult cases
allow anatomic clarification and detection of bile duct
stones or injury-IOC can identify unsuspected common bile duct stones [in roughly 4% of cases, stones
are found on IOC in some series], allowing them to be
dealt with in the same setting (via CBD exploration or
postoperative ERCP) [43]. It can also show a leak or
extravasation if a partial injury has occurred, enabling
immediate repair.
Guidelines by organizations like SAGES and WSES
support liberal use of IOC, particularly in high-risk
cases. A large meta-analysis by Donnellan et al. of
62 studies concluded IOC is a useful tool with a high
detection rate of abnormalities and can be done selectively based on risk factors [43]. Critics of routine
IOC note it can prolong operative time and that there
is no Level-I evidence proving it reduces the incidence
of bile duct injury. However, some population studies
have observed lower BDI rates at institutions with a
policy of routine IOC, suggesting it may serve as a
safeguard. Cholangiography remains a widely used
adjunct to enhance safety, and every difficult cholecystectomy should include consideration of an IOC
at the very least. In some healthcare systems, IOC is
done routinely on all cholecystectomies as standard
practice.
- 3. ICG Fluorescence Cholangiography:
A notable recent advance in biliary surgery is the use
of indocyanine green [ICG] fluorescence imaging to
visualize bile ducts intraoperatively. ICG is a nontoxic
dye that, when injected intravenously [usually 0.25–
0.5 mg/kg a few hours or minutes before surgery], is
taken up by the liver and excreted into bile. Using a
near-infrared [NIR] camera system on laparoscopic or
robotic equipment, the biliary tree can be seen glowing [fluorescing] in real-time during the operation. Several
studies suggest that ICG enhances identification
of extrahepatic bile ducts and can help avoid misidentification injuries [44-46]. Notably, unlike an IOC which
is typically done after dissection has started, ICG can
outline ducts before any dissection – sometimes the
cystic duct is seen entering the common duct as a
fluorescent structure. A systematic review of ICG use
found that it reduced conversion rates (0.5% vs 2.5%
in non-ICG cases) and had a lower incidence of bile
duct injury (0.12% vs 1.3%) in a pooled analysis [45].
Another study in patients with acute cholecystitis after
percutaneous gallbladder drainage showed ICG fluorescence guidance significantly shortened operative
time and drastically lowered conversion to open (2.6%vs 22% without ICG) [47]. These are promising results,
indicating that fluorescence cholangiography can be
a real asset in difficult cases by making the invisible
anatomy visible. Early studies and meta- analyses
have shown reductions in operative time, conversion,
and possibly bile duct injury when ICG is used.
- Artificial Intelligence:
Although in early stages, research is underway on machine learning
tools that could, for example, highlight
biliary anatomy on the surgeon’s screen automatically
or warn when dissection is nearing a critical structure.
Some experimental systems already attempt automatic identification of the cystic duct/artery on the laparoscopic
feed [48, 49]. While not yet in clinical use
widely, these technologies represent the next frontier
in preventing errors.
Conclusion
Challenging cholecystectomy scenarios demand a
high level of surgical awareness, flexibility, and adherence to safe principles. Surgeons must know their
hepatobiliary anatomy, employ sound intraoperative
strategies, and be willing to adjust the operative plan
when managing severe cholecystitis, aberrant anatomy, or other challenging gallbladder conditions.
Key steps include obtaining the critical view of safety, utilizing cholangiography or modern fluorescence
techniques to clarify anatomy, and recognizing when
to bailout. With continued emphasis on education,
research, and adoption of best practices worldwide,
the management of challenging gallbladder cases will
continue to improve, hopefully driving complications
and conversion rates ever lower.
References
- Abdallah, Hamdy S., Mohamad H. Sedky, and Zyad H. Sedky. , “The difficult
Laparoscopic Cholecystectomy: a narrative review.” BMC surgery 25, no. 1 (2025): 156.
- Sugrue, Michael, Shaheel M. Sahebally, Luca Ansaloni, and Martin D. Zielinski.
“Grading operative findings at Laparoscopic Cholecystectomy-a new scoring system.”World Journal of
Emergency Surgery 10 (2015): 1-8.
- Simorov, Anton, Ajay Ranade, Jeremy Parcells, Abhijit Shaligram, Valerie Shostrom, Eugene
Boilesen, Matthew Goede, and Dmitry Oleynikov.
“Emergent cholecystostomy is superior to open cholecystectomy in extremely ill patients with acalculous
cholecystitis: a large multicenter outcome study.”
The American Journal of Surgery 206, no. 6 (2013): 935-941.
- Laws, Henry L.“The difficult cholecystectomy: problems during dissection and
extraction.”
In Seminars in Laparoscopic Surgery, vol. 5, no. 2, pp. 81-91. Sage CA: Thousand Oaks, CA: Sage Publications,
1998.
- Michael Brunt, L., Daniel J. Deziel, Dana A. Telem, Steven M. Strasberg, Rajesh Aggarwal,
Horacio Asbun, Jaap Bonjer et al.
“Safe cholecystectomy multi-society practice guideline and state-of-the-art consensus conference on
prevention of bile duct injury during cholecystectomy.”
Surgical endoscopy 34 (2020): 2827-28
- Bat, Orhan.“The analysis of 146 patients with difficult Laparoscopic
Cholecystectomy.“International journal of clinical and experimental medicine 8, no. 9 (2015): 16127.
- Kulen, Fatih, Deniz Tihan, Uğur Duman, Emrah Bayam, and Gökhan Zaim.“Laparoscopic
partial cholecystectomy: A safe and effective alternative surgical technique in “difficult
cholecystectomies“Turkish Journal of Surgery/ Ulusal Cerrahi Dergisi 32, no. 3 (2016): 185.
- Agrawal, Nikhil, Sumitoj Singh, and Sudhir Khichy.“Preoperative prediction of
difficult Laparoscopic Cholecystectomy: a scoring method.”Nigerian Journal of Surgery 21, no. 2 (2015):
130-133.
- Pesce, Antonio, Teresa Rosanna Portale, Vincenzo Minutolo, Roberto Scilletta, Giovanni Li
Destri, and Stefano Puleo.“Bile duct injury during Laparoscopic Cholecystectomy without intraoperative
cholangiography: a retrospective study on 1,100 selected patients.”Digestive surgery 29, no. 4 (2012): 31
- Abdalla, Sala, Sacha Pierre, and Harold Ellis.“Calot’s triangle
”Clinical anatomy 26, no. 4 (2013): 493-501.
- Andall, R. G., Petru Matusz, Maira du Plessis, Robert Ward, R. S. Tubbs, and Marios
Loukas.“The clinical anatomy of cystic artery variations: a review of over 9800 cases.
”Surgical and Radiologic Anatomy 38 (2016): 529-539.
- Gupta, Vishal, and Gaurav Jain.“Safe Laparoscopic Cholecystectomy: Adoption of
universal culture of safety in cholecystectomy.
”World journal of gastrointestinal surgery 11, no. 2 (2019): 62.
- Strasberg, Steven M., Martin Hertl, and Nathaniel J. Soper.“An analysis of the
problem of biliary injury during Laparoscopic Cholecystectomy.
”Journal of the American College of Surgeons 180, no. 1 (1995): 101-125.
- Dahmane, Raja, Abdelwaheb Morjane, and Andrej Starc.“Anatomy and surgical
relevance of Rouviere’s sulcus.”The Scientific World Journal 2013, no. 1 (2013): 254287.
- Asghar, A., A. Priya, N. Prasad, A. Patra, and D. Agrawal.“Variations in
morphology of cystic artery: systematic review and meta-analysis.
”La Clinica Terapeutica 175, no. 3 (2024).
- Kamath, B. Kavitha.“An anatomical study of Moynihan’s hump of right hepatic
artery and its surgical importance.
”Journal of the Anatomical Society of India 65 (2016): S65-S67.
- Bissinde, Evariste, Raffaele Brustia, and Eric Savier.“Early bifurcation of the
common hepatic artery: A pitfall that should be known and recognized.
”Journal of Visceral Surgery (2024).
- Fateh, Omer, Muhammad Samir Irfan Wasi, and Syed Abdullah Bukhari.“Anaotmical
variability in the position of cystic artery during laparoscopic visualization.
”BMC surgery 21 (2021): 1-5.
- Vasiliadis, Konstantinos, Elena Moschou, Sofia Papaioannou, Panagiotis Tzitzis, Albion
Totsi, Stamatia Dimou, Eleni Lazaridou, Dimitrios Kapetanos, and Christos Papavasiliou.“Isolated aberrant
right cysticohepatic duct injury during Laparoscopic Cholecystectomy: Evaluation and treatment challenges of a
severe postoperative complication associated with an extremely rare anatomical variant.
”Annals of Hepatobiliary-pancreatic Surgery 24, no. 2 (2020): 221-227.
- Amar, Asmae Oulad, Christine Kora, Rachid Jabi, and Imane Kamaoui.“The Duct of
Luschka: an anatomical variant of the biliary tree–two case reports and a review of the literature.
”Cureus 13, no. 4 (2021).
- Spanos, Constantine P., and Theodore Syrakos.“Bile leaks from the duct of Luschka
(subvesical duct): a review.
”Langenbeck’s archives of surgery 391 (2006): 441-447.
- Tran, Thomas, and Ikram Kureshi.“Bile Leak from the Duct of Luschka: A
Multidisciplinary Approach: 561.
”Official journal of the American College of Gastroenterology| ACG 106 (2011): S216.
- Beltrán, Marcelo A.“Mirizzi syndrome: history, current knowledge and proposal of
a simplified classification.
”World journal of gastroenterology: WJG 18, no. 34 (2012): 4639.
- Lai, Eric CH, and Wan Yee Lau.“Mirizzi syndrome: history, present and future
development.
”ANZ journal of surgery 76, no. 4 (2006): 251-257.
- Valderrama-Treviño, Alan Isaac, Juan José GranadosRomero, Mariana Espejel-Deloiza,
Jonathan ChernitzkyCamaño, Baltazar Barrera Mera, Aranza Guadalupe Estrada-Mata, Jesús Carlos Ceballos-Villalva,
Jonathan Acuña Campos, and Rubén Argüero-Sánchez. “Updates in Mirizzi syndrome.” Hepatobiliary surgery and
nutrition 6, no. 3 (2017): 170.
- Csendes, A., J. Carlos Diaz, P. Burdiles, F. Maluenda, and O. Nava.“Mirizzi
syndrome and cholecystobiliary fistula: a unifying classification.
”British Journal of Surgery 76, no. 11 (1989): 1139-1143.
- Ayoub, R., O. Al-Shweiki, H. Bari, V. B. Giriradder, and A.
Mohamedahmed.“Management of Complex Gallstone Disease with Bouveret’s and Mirizzi Syndrome: A Case Report
with Literature Review.
”J Clin Med Surgery 5, no. 1 (2025): 1185.
- VandenDriessche, V., P. Yengue, J. Collin, and M. Lefebvre.“Combination therapy
based on SpyGlass-guided electrohydraulic lithotripsy through cholecystoduodenostomy by lumen-apposing metal
stent (SLAMS) for Mirizzi syndrome.
”Acta GastroEnterologica Belgica 87 (2024).
- Strasberg, Steven M., and Michael L. Brunt.“Rationale and use of the critical
view of safety in Laparoscopic Cholecystectomy.
”Journal of the American College of Surgeons 211, no. 1 (2010): 132-138.
- Booij, Klaske AC, Philip R. de Reuver, Bram Nijsse, Olivier RC Busch, Thomas M. van
Gulik, and Dirk J. Gouma.“Insufficient safety measures reported in operation notes of complicated
laparoscopic cholecystectomies.
”Surgery 155, no. 3 (2014): 384-389.
- Avgerinos, C., D. Kelgiorgi, Z. Touloumis, L. Baltatzi, and C. Dervenis.“One
thousand laparoscopic cholecystectomies in a single surgical unit using the “critical view of safety” technique.
”Journal of Gastrointestinal Surgery 13 (2009): 498-503.
- Strasberg, Steven M.“A three‐step conceptual roadmap for avoiding bile duct
injury in Laparoscopic Cholecystectomy: an invited perspective review.
”Journal of Hepato‐Biliary‐Pancreatic Sciences 26, no. 4 (2019): 123-127.
- Elshaer, Mohamed, Gianpiero Gravante, Katie Thomas, Roberto Sorge, Salem Al-Hamali, and
Hamdi Ebdewi.“Subtotal cholecystectomy for “difficult gallbladders”: systematic review and meta-analysis.
”JAMA surgery 150, no. 2 (2015): 159-168
- Strasberg, Steven M., Michael J. Pucci, L. Michael Brunt, and Daniel J.
Deziel.“Subtotal cholecystectomy– “fenestrating” vs “reconstituting” subtypes and the prevention of bile
duct injury: definition of the optimal procedure in difficult operative conditions.
”Journal of the American College of Surgeons 222, no. 1 (2016): 89-96.
- Chavez, M., R. Figueroa, M. A. Mercado, and I. Domínguez.“Bile duct injury
associated morbidity exceeds subtotal cholecystectomy morbidity.
”HPB 21 (2019): S76-S77.
- Gross, Abby, Hanna Hong, Mir Shanaz Hossain, Jenny H. Chang, Chase J. Wehrle,
Siddhartha Sahai, Joseph Quick et al.“Clinical and patient-reported outcomes following subtotal
cholecystectomy: 10-year singleinstitution experience.
”Surgery 179 (2025): 108805.
- Garzali, Ibrahım Umar, Anas Aburumman, Yousef Alsardia, Belal Alabdallat, Saad Wraikat,
and Ali Aloun.“Is fundus first Laparoscopic Cholecystectomy a better option than conventional Laparoscopic
Cholecystectomy for difficult cholecystectomy? A systematic review and meta-analysis.
”Updates in Surgery 74, no. 6 (2022): 1797-1803.
- Le, Viet H., Dane E. Smith, and Brent L. Johnson. “Conversion of laparoscopic to
open cholecystectomy in the current era of laparoscopic surgery.
”The American Surgeon 78, no. 12 (2012): 1392-1395.
- Nassar, Ahmad HM, Hisham El Zanati, Hwei J. Ng, Khurram S. Khan, and Colin
Wood.“Open conversion in Laparoscopic Cholecystectomy and bile duct exploration: subspecialisation safely
reduces the conversion rates.
”Surgical endoscopy (2022): 1-9
- Lunardi, Nicole, Aida Abou-Zamzam, Katherine L. Florecki, Swathikan Chidambaram, I-Fan
Shih, Alistair J. Kent, Bellal Joseph, James P. Byrne, and Joseph V. Sakran.“Robotic technology in
emergency general surgery cases in the era of minimally invasive surgery.
”JAMA surgery 159, no. 5 (2024):493-499.
