Dawit Abdi 1 *, Tadesse Misgana 1 , Abdulselam Asefa 1 , Tilahun Bete 1 , Abdi Temesgen 2 , Abinet Zewudie 2 , Hirko Asefa 1 , Shimelis Tilahun 1 , Jerman Dereje 1
1 Department of psychiatry, Hararamaya University, College of Health and Medical Sciences, Harar, Ethiopia.
2 Bule Hora University, College of Health and Medical Sciences, Department of Psychiatry, Bule Hora, Ethiopia
Correspondence: Dawit Abdi, Department of Psychiatry, College of Health and Medical Science, Haramaya University, Harar, P.O.BOX, 235, Ethiopia, Email: dawitabdibeka@gmail.com
Received: 01 March 2025; Accepted: 31 March 2025; Published: 07 April 2025
Citation: Dawit, Abdi, Tadesse Misgana, Abdulselam Asefa, Tilahun Bete, Abdi Temesgen, Abinet Zewudie, Hirko Asefa, Shimelis Tilahun, Jerman Dereje. “Erectile Dysfunction and Associated Factors among Adult Patients with Hypertension Attending Outpatient Care at Public Hospitals in Harari Regional State, Eastern Ethiopia.” J Healthc Adv Nur (2025): 125 DOI: 10.59462/JHAN.3.1.125
Copyright: © 2025 Abdi D. This is an open-access ar- ticle distributed under the terms of the Creative Com- mons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Introduction: Sexual dysfunction is lack of sexual ideas or thoughts, diminished sexual interest or desire, and unresponsive sexual desire. Sexual dysfunctions is a prevalent issue that can impact both the patients’ and their spouses’ quality of life. Even though Erectile dys- function is very common and linked to many medical and health related conditions. There was no study at public hospitals in Eastern Ethiopia. Objective.This study was aimed to assess the preva- lence of erectile dysfunction and associated factors among hypertensive patients attending Public Hospitals in Harari Region, Eastern Ethiopia.
Materias and Methods: An institutional-based cross-sectional study was conducted from January 31 to February 29,2024 among 413 participants selected by systematic random sampling technique. Erectile dys- function was assessed with the International Index of Erectile Function.Data were entered into EPI Data ver- sion 3.1 and analyzed using STATA version 14. Bivari- able and multivariable logistic regressions were done to identify factors associated with Erectile dysfunction. The adjusted odds ratio [AOR] with a 95% confidence interval [CI] was computed when the p-value was less than 0.05, which was considered statistically significant. Results: A total of 413 male adult hypertensive patients were recruited in this study with a 98.3% response rate. The prevalence of erectile dysfunction among hyperten- sive patients was 54%[95% CI 49.2-58.4]. Age between 61 and 80 years [AOR=3.3,95%CI:1.06-10.32], having a depression [AOR=3.1,95%CI: 1.63- 6.16], having an anxiety [AOR=2.3,95% CI: 1.21- 4.41], being in a stage 2 hypertension [AOR=3.2,95% CI:1.09- 9.77], duration of hypertension more than 10 years [AOR=5.6,95%- CI:1.98-16.06], having comorbid medical illnesses [AOR=4.0,95%, CI:2.13- 7.53], being on antihyperten- sive polytherapy [AOR=3.6,95%CI:1.99-6.51], and be- ing physically inactive [AOR=4.4,95% CI:2.42- 8.07] were significantly associated with erectile dysfunction on multivariable logistic regressions.
Conclusion: More than half of the study participants had erectile dysfunction. and it appears to be signifi- cantly associated with age, presence of depression and anxiety, stage of hypertension, duration of the illness, preexisting chronic illnesses, medication polytherapy, and physical activity.
The inability to obtain or maintain an adequate penile erection for satisfying sexual performance is known as Erectile dysfunction [ED], failure to achieve or maintain a rigid penile erection suitable for satisfactory sexual intercourse. Marked difficulty in obtaining an erection during sexual activity, difficulty in maintaining an erection until the completion of sexual activity. Marked decrease in erectile rigidity. persisted for a minimum duration of approximately 6 months.[1].According to the sexual response cycle, sexual disorders in males are categorized as hypoactive sexual desire, premature ejaculation disorder, erectile dysfunction, and orgasmic dysfunctionn [1] .Different literatures have shown that the prevalence of erectile dysfunction among patients with Hypertension was ranged from 43.2% to 66.2% [2-5].
The World Health Organization defines hypertension as blood pressure more than or equal to 140/90mmHg in an adult. Hypertension causes endothelial dysfunction by the sheer stress of raised blood pressure on the endothelium [6]. Hypertension and erectile dysfunction are closely related conditions that share a common cause of endothelial dysfunction [7]. Endothelial dysfunction impairs the balance between vasoconstrictors and vasodilators, which affects blood flow and the ability to maintain an erection. Erectile dysfunction can be an early sign of hypertension, as well as a consequence of it [8, 9].
Blood flow to all parts of the body, including the penis, is decreased by high blood pressure’s damage to blood vessels [10]. Blood flow into the penis prior to sex is impeded by the penis’s hardened and narrow blood vessels [11]. The penis becomes firm as a result of blood accumulating in the corpus cavernous during an erection. The penis’ muscles relax after an orgasm, allowing blood to flow once more through the body. The result is that the man’s urogenital system reverts to its pre-arousal state, the erection decreased, and the penis becomes limp and soft [11].
Over 30 million men in the United States are affected by erectile dysfunction, with a worldwide estimate of approximately 100 million men affected by this condition. [12]. furthermore, this number is projected to increase to 322 million men by 2025[13], with the largest increases anticipated in Africa, Asia, and South America. In the USA, hypertension, a significant risk factor for ED, affects 29.4 million men [14, 15]. ED affects a large part of the population, increasing its incidence with age and comorbidities [16]. Evidence has shown that ED significantly lowers quality-of-life indicators. ED places a significant financial burden on men’s employers as well as a significant quality of life burden on men and their female partners. Similarly, ED also has a negative effect on a man’s female partners because it causes problems in their relationships and lowers their level of satisfaction. In comparison to men without ED, men with ED exhibit higher rates of absenteeism [impairment while at work], and loss of work productivity, which impose a significant financial burden on employers [17-19]. Erectile dysfunction reduces productivity at work, causes people to avoid intimate relationships, and has an influence on quality of life [20].
Numerous studies have suggested that ED and depression are symbiotically related. Men with depression tend to have an almost 5-fold higher prevalence of ED, and vice versa—men with ED frequently report higher rates of depression [21]. Endothelial dysfunction, atherosclerosis, and adverse effects of antihypertensive medicines are a few of the pathogeneses that link hypertension and ED [7]. Age, stage, and duration of hypertension, antihypertensive drug use, depression, and behavioral factors including alcohol use, smoking, being overweight or obese, and physical inactivity, all have an impact on a patient’s ability to erection [22, 23].
Even though ED is very common and is linked to many medical and health related conditions, it is still underdiagnosed and undertreated [24]. Many developing nations routinely underrate erectile dysfunction [25]. Some (mainly younger men) think that the condition will go away on its own, while others think that ED is a natural part of aging (primarily older men) [26]. There were no studies on ED among adult hypertensive patients at public hospitals in harari region. Therefore, this study aimed to determine the prevalence and associated factors of ED among adult hypertensive patients attending ppublic hospitals in Harari region.
An institution-based cross-sectional study was conducted. The study was conducted at Public Hospitals in the harari region which are found in Harar town. A Harar town, the capital city of Harari regional state, is located in eastern part of Ethiopia and is 526km from Addis Ababa. Harar is with an area of17.20 Km2, 90% of the region is estimated to be woynadega (Between 1000-1500m) and situated at 900, 230 Altitude and 420, 240 longitude and with elevation of 1600 feet above the sea level. The region has 2 public hospitals; named Haramaya University Hiwot Fana Comprehensive Specialized Hospital and Jugol Hospital, 1 federal police hospital, 8 health center, 26 health posts,
According to the data taken from the hospital Number of male patients with hypertension attending medical out-patient care were 480 monthly and 5250 Annually at Haramaya University Hiwot Fana Comprehensive
Specialized Hospital. Jugol hospital is the first hospital founded in Harar town. According to the data taken from the hospital Number of male patients with hypertension attending medical out-patient care were 436 monthly and 4850 Annually at Jugol. Study was conducted from January 31 to February 29, 2024.
Source Population and Study population
Source population: All male patients with hypertension at Public Hospitals in harari region.
Study population: Men adult patients with hypertension those who are selected by systematic sampling technique at Public Hospitals in harari region.
Inclusion criteria: Men adults with hypertension Male adult hypertensive patients who are 18 years of age or older and had sexual activity within the One month before the study
Exclusion criteria: Men adult with hypertension who have undergone surgery for their penile, urethral, or prostate in the past were not included in the study.
Sample size determination: The Sample size of the first objective of this study was calculated by using a single population proportion formula.
The formula employed as: n= [Zα/2]2pq/d2
n=((Zα/2)2×p[P-1])/d2
Where, n= minimum sample size is required for the study.
z=the reliability of coefficient corresponding to 95% confidence level. That means at 95% z value is 1.96.
d= the absolute precision of tolerable margin of error [d= [0.05].
p= the prevalence of ED which is 46.34% in previously done at Gonder city government health facility [22].
n= [Za/2]2 p[1-p]/d2
= [1.96]2 x 0.4634[1-0.4634]/ [0.05]2
= 3.8416 x0.4634 [0.5366]/ 0.0025
n=382
Then by adding 10% of the non-respondent rate.
10x382/100 =38
Total sample size 382+38= 420
Sampling procedure: Systematic sampling techniques was used select study participants. the estimated sample was proportionally allocated to each hospital depending on the number of adult hypertensive patients attending medical out-patient department. There were 480 and 436 adult patients with hypertensive patients who visited Haramaya University Hiwot Fana Comprehensive Specialized Hospital and Jugol hospital per month respectively= 916/ 420 = 2.18~ 2. The study participants were interviewed every 2 intervals at each hospital and the first participant was selected randomly by using simple lottery method (Figure 1).
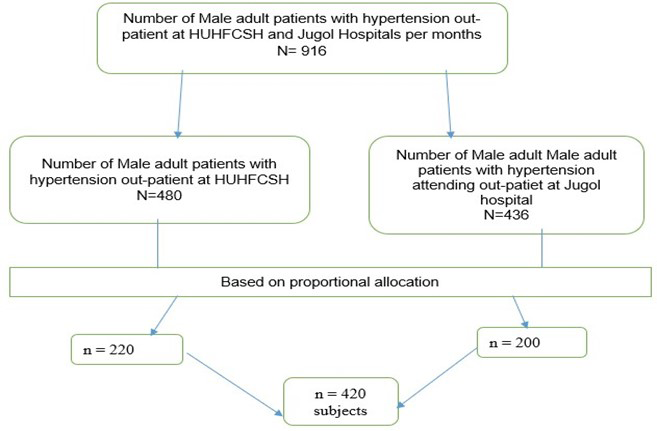
Figure 1: Schematic presentation of sampling procedure for
magnitude of Erectile dysfunction and associated factors among
adult patients with Hypertension attending Follow up care at
public hospitals in harari region, Eastern Ethiopia, 2024.
Dependent variable
Erectile dysfunction [Yes/No]
Independent variables
Sociodemographic variables such as [age, marital status, education, occupation and income], clinical variables [stage of hypertension, duration of hypertension after diagnosis, duration of therapy, antihypertensive drugs], comorbidities [like diabetes, cardiovascular disease, chronic kidney disease, depression, obesity and anxiety].
Operational definition
Erectile dysfunction: According to the International Index of Erectile Function, People with hypertension who scored 5 to 21 out of 25 was classified as having ED. The severity of ED was classified as Mild Erectile Dysfunction [12-21],Moderate Erectile Dysfunction [8-11] and Severe Erectile Dysfunction [5-7] [27].
Depression: Hospital Anxiety Depression Scale score ≥8 is an indicator of depression [29].
Anxiety: Hospital Anxiety Depression Scale score ≥8 is an indicator of anxiety disorder [29].
Physical activity: If a hypertensive man exercises at least 150 minutes a week at a moderate intensity, 75 minutes at a robust intensity, or an equivalent amount of both moderate and vigorous intensity, he was physically active [30].
BMI of study participants was measured by dividing their weight in kilograms by the square of their height in meters and interpreted as underweight [<18.5 kg/m2], normal [18.5–24.9 kg/m2] and overweight [≥25 kg/m2] [14].
Current substance use: Using at least one of a specific substance for nonmedical purpose within last 3 months [31].
Ever substance use: using at least one of any specific substance for the nonmedical purpose at least once in a lifetime [31].
Monthly income: World Bank established international poverty line as 1.9 USD. Converting to Ethiopian context as monthly income, [1.9*56.56*30= 3,224 Ethiopian Birr] monthly income <3224 Ethiopian Birr was considered as below poverty line [32].
Data collection Tools and procedures
Three BSc psychiatric professionals and Four BSc nurses were involved in data collection with close supervision by principal investigator and supervised by two MSc psychiatry professional’s Erectile dysfunction was dependent variable and numerous additional independent
variables, including sociodemographic characteristics such as age, marital status, income, clinical factors, comorbid factors, and substance use were gathered using a pretested interviewer-administered questionnaire. The time since hypertension was diagnosed and started taking antihypertensive medications and the presence of comorbidities were taken from the patient’s medical records.
The presence and severity of ED were assessed using an interviewer-administered International Index of Erectile Function. The International Index of Erectile Function [IIEF-5] instrument, a short, reliable, and valid questionnaire deemed effective in the clinical assessment of sexual activities and treatment outcomes in clinical trials. The tool provides scoring of patients with the lowest score being 5 and the highest 25. The higher the score, the less the severity of ED. A score of above 21 indicates no ED with 98% sensitivity and 88% specificity. The severity of ED was classified as Mild Erectile Dysfunction [12-21],Moderate Erectile Dysfunction [8-11] and Severe Erectile Dysfunction [5-7]. The Cronbach’s alpha values of IIEF was 0.89.The reliability of this instrument using Cronbach’s Alpha in this study was 0.85 [27].
The study participants’ weight was measured and recorded in kilograms. Height was measured using a stadiometer to the nearest 0.5 centimeters. The study participants’ body mass index was calculated and interpreted as underweight [18.5 kg/m2], normal[18.5-24.9 kg/m2], or overweight [>24.9 kg/m2] [14].
The staging of hypertension was categorized according to the JNC-8 guideline. After the participant has rested for at least five minutes and has abstained from smoking and caffeine for 30 minutes prior to measurement, their blood pressure was measured twice while they are seated using a standard Mercury sphygmomanometer BP cuff. The blood pressure was calculated using the average of two readings taken five minutes apart [28].
Hospital anxiety and depression scale [HADS] was used to assess anxiety and depression. It has two subscales: the anxiety subscale [HADS-A] and the depression subscale [HADS-D]. Each subscale contains seven items for a total of 14 items in the HADS. It has cutoff point ≥ 8 for each
subscale. It was validated in Ethiopia among HIV infected patients. The Cronbach’s Alpha was 0.87 for the full scale of HADS.Cronbach’s Alpha in this study was 0.88 [29].
The global physical activity questionnaire suggested by WHO was used to assess physical activity [30].
Data quality control and management
The questionnaire was pretested on 5% male hypertensive patients attending their follow-up at Haramaya general hospital and the questionnaire was revised and amended. Training was given to data collectors for two days by investigator, data collectors were supervised at each site, regular meetings was held between the data collector, supervisor, and the principal investigator. The collected data were reviewed and checked for completeness by supervisors and principal investigator each day after data collection. English version of the questionnaire was translated to Amharic version and Afan Oromo version then translated back to English version by language expert. privacy of patient was always be maintained during interviewing to make sure the data provided is reliable.
Data analysis
The data were checked for completeness and consistency then the data were coded and entered into Epi-data version 3.1 and analyzed by using STATA version14. Then, the data were analyzed to generate descriptive statistics: means, frequency, percentages, and summery measures. Bivariable and multivariable logistic regressions was used to identify the independent factors of ED. This was done by entering each independent variable separately into bivariate analysis. Then, variables with p-value of less than 0.25 on bivariable analysis were entered into multivariable logistic regression. The results of multi-variaable logistic regression analysis were presented in crude and adjusted odds ratios with 95% confidence intervals. The level of statistical significance was declared at a P-value < 0.05. The strength of association was determined using
Adjusted odds ratios with 95% confidence interval. The fitness of the model was checked using Hosmer and Lemeshow test p- value 0.53, and multicollinearity was checked by using variance inflation factor of <10 and tolerance of >0.1.
The study was carried out under consideration of the Helsinki Declaration of medical research ethics Ethical approval was obtained from the Institutional Health Research Ethics Review Committee [IHRERC] of Haramaya University, college of health and medical sciences [Ref.No. IHRRC/034/2024]. Co-operation letters was written to administrative bodies of the Haramaya University Hiwot Fana Comprehensive Specialized Hospital and Jugal Hospital asked for their permission of the research to be conducted in the institution. Informed, voluntary, written [finger print for those unable to read and write] and signed consent were obtained from each participant before data collection. Participants were informed about the aim of study and advantage of study; to secure confidentiality of participants’ all information that was collected, kept confidential and not accessed to any third party. privacy of patient was always maintained during interviewing to make sure the data provided is reliable.
Socio-demographic characteristics of the study participants
A total of 413 male adult hypertensive patients were recruited in this study with a 98.3% response rate. The mean age of the respondents was 57.01 (±13.9) years. The majority 348 (84.26%) of the participants were married,248 (60.05%) were Oromo in ethnicity, and 281 [68.04%] were Muslim in religion. Regarding the level of education,115 (27.85%) had attained college and above (Table 1).
Table 1: Sociodemographic characteristics of hypertensive patients attending outpatient care at public hospitals in Harari region, Eastern Ethiopia,2024[n=413]
| Variable | Category | Frequency | Percent [%] |
|---|---|---|---|
| Age group [years] | 18–40 | 52 | 12.59 |
| 41–60 | 204 | 49.39 | |
| 61–80 | 120 | 29.06 | |
| Above 80 | 37 | 8.96 | |
| Marital status | Single | 32 | 7.75 |
| Married | 348 | 84.26 | |
| Divorced | 13 | 3.15 | |
| Widowed | 20 | 4.84 | |
| Ethnicity | Oromo | 248 | 60.05 |
| Amhara | 119 | 28.81 | |
| Harari | 20 | 4.84 | |
| Somali | 12 | 2.91 | |
| Others* | 14 | 3.39 | |
| Religion | Muslim | 281 | 68.04 |
| Orthodox | 106 | 25.67 | |
| Protestant | 15 | 3.63 | |
| Others** | 12 | 2.67 | |
| Educational level | Unable to read/write | 62 | 15.01 |
| Able to read/write | 53 | 12.83 | |
| Primary school | 92 | 22.28 | |
| Secondary school | 91 | 22.03 | |
| College and above | 115 | 27.85 | |
| Occupation | Farmer | 96 | 23.24 |
| Merchant | 106 | 25.67 | |
| Government employee | 80 | 19.37 | |
| NGO employee | 70 | 16.95 | |
| Daily laborer | 16 | 3.87 | |
| Retired | 45 | 10.90 | |
| Monthly income | <3,224 ETB | 109 | 26.39 |
| >3,224 ETB | 304 | 73.61 |
Clinical characteristics of study participants
Among the study participants, 142 [34.38%] patients had controlled their blood pressure.The mean body mass index [BMI] was 21.78 ± 2.5 kg/m2. Regarding the duration of hypertension, 81[19.61% ] study participants lived with hypertension more than 10 years.Among hypertensive patients, 221 [53.51%] were on polytherapy and 88
[21.3 %] were on diuretics. Among study participants
317 [76.76%] had normalweight and 191 [46.25%] had comorbid medical illness. The most common comorbid medical illness was diabetes mellitus, which accounted for 99 [23.97%], followed by cardiovascular disease 69 [16.71 %]. In this study, the prevalence of depression was 38.50% and prevalence of anxiety was 39.47% (Table 2).
Table 2. Clinical characteristics of male adult hypertensive patients attending public hospitals in Harari region, Eastern Ethiopia,2024 [n=413]
| Variable | Category | Frequency | Percent [%] |
|---|---|---|---|
| Stage of hypertension | Controlled (<140/90) | 142 | 34.38 |
| Stage 1 (140–159/90–99) | 223 | 54.00 | |
| Stage 2 (>160/100) | 48 | 11.62 | |
| Duration of hypertension | <5 years | 172 | 41.65 |
| 5–10 years | 160 | 38.74 | |
| >10 years | 81 | 19.61 | |
| Body Mass Index (kg/m²) | Underweight | 17 | 4.12 |
| Normal | 317 | 76.76 | |
| Overweight | 79 | 19.13 | |
| Antihypertensive therapy | Monotherapy | 192 | 46.49 |
| Polytherapy | 221 | 53.51 | |
| Medication class | Diuretics | 88 | 21.31 |
| Calcium channel blockers | 47 | 11.38 | |
| ACE inhibitor | 42 | 10.17 | |
| Beta blockers | 15 | 3.63 | |
| Diuretics + CaCB | 67 | 16.22 | |
| Diuretics + BB | 46 | 11.14 | |
| CaCB + ACEI | 42 | 10.17 | |
| Diuretics + CaCB + BB | 66 | 15.98 | |
| Comorbidities | Yes | 191 | 46.25 |
| No | 222 | 53.75 | |
| Types of comorbidities | Diabetes mellitus | 99 | 23.97 |
| Cardiovascular disease | 69 | 16.71 | |
| Chronic kidney disease | 17 | 4.12 | |
| Others* | 6 | 1.45 | |
| Depression | Yes | 159 | 38.50 |
| No | 254 | 61.50 | |
| Anxiety | Yes | 163 | 39.47 |
| No | 250 | 60.53 |
Behavioral characteristics of study participants
Among the total of 413 respondents,243 [58.84%] were
life time khat chewer, while 221 [53.51%] were current
khat chewer. Among participants,47[11.38%] of study participants were life time alcohol drinker and 41[9.93%] were current alcohol drinker. Among respondents, 80[19.37%] were life time cigarette smoker and 71[17.97%] were current cigarette smoker (Table 3).
Table 3. Behavioral characteristics of male adult hypertensive patients attending public hospitals in Harari region, Eastern Ethio- pia,2024[n=413].
| Variable | Category | Frequency | Percent [%] |
|---|---|---|---|
| Lifetime khat chewer | Yes | 243 | 58.84 |
| No | 170 | 41.16 | |
| Current khat chewer | Yes | 221 | 53.51 |
| No | 192 | 46.49 | |
| Lifetime alcohol drinker | Yes | 47 | 11.38 |
| No | 366 | 88.62 | |
| Current alcohol drinker | Yes | 41 | 9.93 |
| No | 372 | 90.07 | |
| Lifetime smoker | Yes | 80 | 19.37 |
| No | 333 | 80.63 | |
| Current smoker | Yes | 71 | 17.19 |
| No | 342 | 82.81 | |
| Physical activity | Yes | 222 | 53.75 |
| No | 191 | 46.25 |
Prevalence of erectile dysfunction among hypertensive patients
The prevalence of ED was 54% [95% CI 49.2-58.4]. The distribution of ED was mild in 105[25.42%, 95% CI 21.5-29.9] moderate in 90[21.79%,95%CI 17.7-25.7] and severe in 28[6.78%,95% CI 4.4-9.2] study participants (Figure 2).
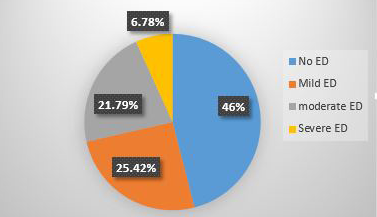
Figure 2. Prevalence of erectile dysfuncton among hypertensive patients attending public hospitals in Harari region,Eastern Ethiopia,2024
Factors associated with erectile dysfunction among hypertensive patients
In the bi-variable logistic regression analysis, variables such as age, duration of hypertension, stage of hypertension, body mass index, lifetime khat use, current khat use, depression, anxiety, comorbid medical illness, Antihypertensive polytherapy, physical activity with p value less than 0.25 were selected for multivariable logistic regression analysis. On the final model the result showed that age, duration of hypertension, depression, anxiety, comorbid medical illness, Antihypertensive polytherapy, stage of hypertension, and physical activity
were significantly associated with erectile dysfunction at p-value <0.05. In this study, the odds of having erectile dysfunction were 3.3 times higher for patients aged between 61 and 80years,[AOR=3.3,95%CI: 1.06-10.32] and 6.1 times higher for those above 80years[AOR=6.1,95%CI: 1.19- 31.97] compared with those aged between 18 and 40 years. This study also showed that the odds of having erectile dysfunction was 3.1 times [AOR=3.1,95%CI: 1.63- 6.16]. and 2.3 times [AOR=2.3,95%CI: 1.21- 4.41] higher among participants who had depression and anxiety as compared to respondents who had no depression and anxiety respectively.
In this study,the odds of having erectile dysfunction were 3.2 times higher for those with stage 2 hypertension [AOR=3.2,95%CI:1.09- 9.77] compared with those respondents with controlled hypertension.The odds of having erectile dysfunction were 5.6 times higher for those who had hypertension for more than 10 years [AOR=5.6,95%CI:1.98-16.06] compared with those participants who had hypertension for less than 5 years. This study found that the odds of having erectile dysfunction were 4 times higher in those respondents who had comorbid medical illness [AOR=4,95%CI:2.13- 7.53] compared with those hypertensive patients who had no comorbid medical illness. The odds of having erectile dysfunction were 3.6 times higher in those who were on antihypertensive polytherapy [AOR=3.6,95%CI: 1.99- 6.51] compared to those who were on monotherapy. This study found that the odds of having erectile dysfunction were 4.4 times higher for those physically inactive [AOR=4.4,95%CI:2.42- 8.07] compared with those respondents physically active (Table 4).
Table 4. Bivariable and multi variable logistic regression analysis of factors associated with erectile dysfunction among hyperten- sive patients attending public hospitals in harari region, Eastern Ethiopia,2024 [n=413]
| Variable | Erectile Dysfunction Yes [%] | No [%] | COR [95% CI] | AOR [95% CI] |
|---|---|---|---|---|
| Age group [years] | 18–40 (13 [25]) | 39 [75] | 1 | 1 |
| 41–60 | 97 [47.5] | 107 [52.45] | 2.7 [1.37–5.39] | 2.5 [0.90–7.45] |
| 61–80 | 81 [67.5] | 39 [32.5] | 6.2 [2.98–12.99] | 3.3 [1.06–10.32] |
| Above 80 | 32 [86.49] | 5 [13.51] | 19.2 [6.18–59.57] | 6.1 [1.19–31.97]* |
| Stage of hypertension | Controlled 47 [33.09] | 95 [66.90] | 1 | 1 |
| Stage 1 | 138 [61.89] | 85 [38.11] | 3.2 [2.10–5.10] | 1.5 [0.81–3.06] |
| Stage 2 | 38 [79.17] | 10 [20.83] | 7.6 [3.52–16.74] | 3.2 [1.09–9.77]* |
| Depression (Yes vs No) | 124 [77.99] | 35 [22.01] | 5.5 [3.52–8.71] | 3.1 [1.63–6.16]** |
| Anxiety (Yes vs No) | 122 [74.85] | 41 [21.15] | 4.3 [2.84–6.78] | 2.3 [1.21–4.41]* |
| Duration of hypertension | <5 years 68 [39.54] | 104 [60.46] | 1 | 1 |
| 5–10 years | 88 [55] | 72 [45] | 1.8 [1.20–2.89] | 1.0 [0.52–1.91] |
| >10 years | 67 [82.72] | 14 [17.28] | 7.3 [3.81–14.04] | 5.6 [1.98–16.06]** |
| Comorbidity (Yes vs No) | 130 [68.07] | 61 [31.93] | 2.9 [1.97–4.42] | 4.0 [2.13–7.53]** |
| Antihypertensive therapy | Polytherapy 171 [77.38] | Monotherapy 50 [22.62] | 9.2 [5.88–14.41] | 3.6 [1.99–6.51]** |
| Physical inactivity | 140 [73.3] | 51 [26.7] | 4.5 [3.01–6.99] | 4.4 [2.42–8.07]** |
This study aimed to determine the prevalence of ED and associated factors among hypertensive patients who have follow-ups at public hospitals in harari region. The prevalence of ED in this study was 54%[95% CI 49.2- 58.4].Age, duration of hypertension, depression, anxiety,
comorbid medical illness, Antihypertensive polytherapy, stage of hypertension, and physical inactivity were significantly associated with erectile dysfunction.
This result is similar with the study conducted in Cameroon [50.6%] [2] and Thailand [56.6%][33]. However, it was lower than those studies from Qatar [66.2%] [34],
Spain [71.0%][35],South West Nigeria[75.5%] [5] and Kenya [94.5%] [4]. This difference might be due to the sociocultural differences in which cultural factors can influence perceptions and reporting of sexual health issues, including ED. The participants in this study may have different attitudes towards discussing and seeking treatment for ED compared to countries like Qatar, Spain, and Kenya. The result of this study was lower than those of the study conducted in Qatar, which was based on a matched case-control design utilizing the International Index of Erectile Function [IIEF-5] questionnaire. However, a notable difference lies in the choice of cutoff points. While the study conducted Qatar used a cutoff point of 1, our study utilized the IIEF-5 questionnaire with a cutoff point of 5. Consequently, these differences in methodology have led to discrepancies in the result.
And this result was higher than studies from Greece [35.2%] [23] , Israel [21.8%][36], Nigeria [41.5%][37] and Gonder [46.34%] [22]. This difference might be due to the sociocultural differences, Cultural factors can influence perceptions and reporting of sexual health issues, including ED. The study conducted in Gonder included hypertensive patients who had engaged in sexual activity within the three months prior to the study. However, our study included participants who had engaged in sexual activity within the one month preceding the study. While the study conducted in Gonder utilized the International Index of Erectile Function [IIEF-5] questionnaire with a cutoff point of 6, our study used the IIEF-5 questionnaire with a cutoff point of 5. These variations in both the timeframe for sexual activity inclusion and the cutoff points utilized in the questionnaire have resulted in differences between the two-study finding. The international index of erectile function [IIEF] and the sexual inventory for men are the the two commonly used to assess erectile dysfunction,but they have differences in their design,questions,and scoring systems.These can lead to variations in the prevalence of erectile dysfunction among hypertensive patients.The IIEF covers various aspects of sexual function beyond just erectile function such as libido and satisfaction,while the SIM focus more specifically on erectile function. Additionally,differences in the population studied and settings in which the tools are administered can also contribute to discrepancies in prevalence rate In this study being in old age was significantly associated with ED. These results was similar to the study conducted in Qatar [34] Thailand [38]Cameroon [2], Greece [23],Gonder, Ethiopia[22]. Growing older is frequently accompanied by some organic disorders that might impair erection[39].Specifically, age-related ED largely caused by cardiovascular and metabolic disorders, which are more common in older adults [40]. The primary cause of vascular endothelium degenerative changes and endothelial dysfunction in the aging process is the impairment of penile erection, as it is essentially a vascular event. The relaxation process of smooth muscle cells in the corpus cavernosum and small artery wall is significantly reduced, which in turn causes a change in vascular tone[9]. The higher occurrence of ED may possibly be caused by an age-related decline in testosterone levels [41]. The quantity of functional corporal smooth muscles is known to decline with age. It’s thought that the apoptotic process, which is mostly brought on by oxidative stress, is the primary mechanism responsible for the age-related decrease of normal smooth muscle in the body [42].
This study showed that long duration of hypertension was associted with erectile dysfunction. This finding is in line with other studies from Thailand [33], Qatar [34], Israel [36], Greece [43],Cameroo [2],Egypt [44],Gonder [22]. This finding can be explained by the progression of atherosclerosis and endothelial dysfunction with increasing duration and severity of hypertension[45]. The mechanism by which stage 2 hypertension cause ED is likely related to endothelial dysfunction associated with hypertension [46].Long-standing hypertension cause oxidate stress, endothelial cell injury and its sequella, including the inability of the arteries, arterioles and sinusoids of the corpus cavernosum to dilate properly [47]. Long-term hypertension damages artery walls, causing them to harden and narrow, and reducing blood flow to the penis [8]. Nitric oxide [NO] generation by the endothelium is decreased due to endothelial dysfunction, which is linked to hypertension and ultimately causes ED [48]
In this study anti hypertensive polytherapy was significantly associated with ED.This study was in line with studies from Qatar [34],Cameroon [2],Gonder [22]. The erectile dysfunction caused by antihypertensive polytherapy, especially the nonselective, is because of their direct effects on penile vascular smooth muscle
cells causing vasoconstriction from α-adrenergic stimulation leading to decreased perfusion in the corpora cavernosa, Furthermore, polytherapy increases the drug interaction, which can exacerbate ED, combining a beta blocker with diuretic result in additive effects on sexual function [49]. Central-acting beta-blockers and diuretics are known to frequently cause ED because they lower blood pressure and cardiac output, which in turn reduces blood supply to the penile vasculature,antihypertensive polytherapy can cause erectile dysfunction through various mechanisms,including directs effects on vascular function,hormonal changes and drug interaction [50].
In the current study, comorbid medical illnesses were associated with erectile dysfunction. This finding was similar with other studies conducted in France [51],Israel [52],Nigeria [37],Gonder [22]. The most common comorbid condition among study participants was diabetes mellitus. It has an impact on endothelial cells and corpus cavernous nerve terminals, which reduces the production and release of NO and leads to ED [53]. High blood sugar over a long period of time can damage the nerves and blood vessels. This damage causes problems with getting or keeping an erection [54].
This study showed that depression was associated with ED. This finding was similar with other studies conducted in Spain [35], China [55], Finland [56], Gonder [22]. Depression is frequently associated with decreased libido, diminished erectile function, and decreased sexual activity[57]. Depression inhibit the activity of parasympathetic nerves, thereby decreasing the inflow of blood to the penis and inhibiting penile smooth muscle relaxation [56].Depression affects sexual activity due to depletion of neurotransmiters like serotonin, which are chemicals in the brain. These neurotransmitters play a crucial role in regulating both mood and sexual function [58].
Moreover, this study found that ED was significantly associated with anxiety. This study was in line with study conducted in Taiwan [59], Japan [60], China [61]. The role of anxiety in ED has not been clearly established, however, it is proposed that anxiety contributes to a vicious cycle that impairs the sexual relation between the patient and partner resulting in communication problems, which further impede sexual functioning [62]. Anxiety response causes an increase in sympathetic tone, resulting in a distraction from erotic stimuli leading to impaired arousal and erection [63].
This study found that physically inactive was associated with erectile dysfunction. This finding is in line with other studies from Qatar [34], Nigeria [64], Pakistan, Egypt, and Nigeria [65], Gonder [22]. Physical inactivity is strongly linked to poor cardiovascular health, including conditions such as hypertension, atherosclerosis, and coronary artery disease. These conditions can impair blood flow to the penis, which is essential for achieving and maintaining an erection [66]. Physical inactivity can lead to endothelial dysfunction, characterized by impaired production of nitric oxide [NO], a vasodilator that plays a key role in penile erection. Endothelial dysfunction reduces the ability of blood vessels to dilate properly, thus limiting blood flow to the penis and contributing to ED [67]. Physical inactivity is associated with alterations in hormone levels, including decreased testosterone and increased cortisol levels. Testosterone is crucial for maintaining erectile function, libido, and overall sexual health. Low testosterone levels resulting from physical inactivity can contribute to ED [68].
Limitations of the Study
The cross-sectional nature of the study does not allow making inferences about the causal relationship. The study may have social desirability bias due to the type of data collection technique [face-to-face interviewer- administered questionnaire]. inability to measure other confounders, such as testosterone levels.
In the current study, the prevalence of Erectile dysfunction was found to be higher among Hypertensive patients. Age, duration of hypertension, depression, anxiety, comorbid medical illness, Antihypertensive polytherapy, stage of hypertension, and physical activity were associated with erectile dysfunction among Hypertensive patients. All men with hypertension presenting to health care professionals should have routine evaluation for ED so as to recognize it early and reduce its effects. Since ED could be associated with hypertension and other medical illnesses, the finding of ED should prompt the doctor to do a thorough screening of the patient especially for cardiovascular disease.
Conflict of Interests
insightful criticism and ongoing assistance with this paper at every stage. Next, we would like to thank the study participants for their enthusiastic engagement in our research.
improvement in all domains of the PAQLQ. An effective and sustainable school-based asthma education program requires care coordination and team collaboration. This project supports the role of the school nurse in implementing an asthma education program. Future research is needed to examine strategies and resources to support school-based asthma education programs.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request via (dawitabdibeka@gmail.com).
My sincere gratitude goes out to my co-authors for their insightful criticism and ongoing assistance with this paper at every stage. Next, we would like to thank the study participants for their enthusiastic engagement in our research.
improvement in all domains of the PAQLQ. An effective and sustainable school-based asthma education program requires care coordination and team collaboration. This project supports the role of the school nurse in implementing an asthma education program. Future research is needed to examine strategies and resources to support school-based asthma education programs.